Several species of stink bugs are pests of cotton in the southeastern United States, including southern green stink bug, Nezara viridula, green stink bug, Chinavia hilaris (formerly Acrosternum hilare), brown stink bug, Euschistus servus, and brown marmorated stink bug, Halyomorpha halys. Of this complex, brown stink bug, southern green stink bug, and green stink bug are historically the most commonly occurring species in cotton.1 The brown marmorated stink bug is an invasive pest that has increased in prevalence in cotton in South Carolina in recent years. Stink bugs are highly polyphagous (i.e., able to feed on a wide range of hosts) and mobile pests that feed on numerous cultivated crops in the Southeast, including corn, soybeans, peanut, winter wheat, and cotton. It is important to note that, in addition to these pests, there are beneficial, predatory stink bugs in cotton that eat insects and can be confused with pest species of stink bugs, emphasizing the importance of proper identification. Historically, stink bugs were managed in cotton incidentally by frequent insecticide applications targeting the boll weevil, Anthonomus grandis grandis, bollworm, Helicoverpa zea, tobacco budworm, Heliothis virescens, and other pests. Following eradication of the boll weevil and the introduction of transgenic cotton varieties expressing toxins from Bacillus thuringiensis (Bt cotton), broad-spectrum insecticide applications were reduced dramatically in cotton production, resulting in an increase in the frequency of damaging levels of stink bug populations.2 The purpose of this publication is to provide growers, consultants, and other agricultural professionals with background information on stink bugs, describe their injury to cotton, and discuss management strategies.
Identification and Life Cycle
The southern green, green, brown, and brown marmorated stink bugs belong to the family Pentatomidae and undergo similar development and life cycles. In most of the southeastern United States, including South Carolina, stink bugs are bivoltine (i.e., two generations per year). Each species develops from eggs through five nymphal developmental stages (i.e., instars) to an adult and overwinters as adults in various locations, including crop residue, woodlands, leaf litter, and structures. Adults of all species are oval or shield-shaped with a well-developed scutellum, piercing-sucking mouthparts, and five-segmented antennae. First instars of each species feed and aggregate on the hatched egg mass until after the first molt, when second instars begin to disperse.3,4 After emergence from overwintering sites, adults generally feed on early season wild or cultivated hosts, such as winter wheat or field corn, prior to moving into cotton later in the season when developing bolls become available.5 Stimulated by rising temperatures and increasing day length, emergence of adults generally begins in South Carolina from late March through April, depending on the species.6
Southern Green Stink Bug, Nezara viridula
Eggs of the southern green stink bug are cylindrical in shape, pale yellow or cream colored, and are deposited in clusters of sixty to ninety eggs per mass.5 The incubation period of eggs ranges from five days to as much as a couple of weeks, depending on the time of year.3 Nymphs of the southern green stink bug can vary widely in appearance, even within the same nymphal stage. First instars are reddish in color immediately after hatching and turn almost entirely black after the first molt (figure 1). Second and third instars are black with spots of white and red on the abdomen. Fourth and fifth instars are primarily either green or yellowish-brown and have a series of distinct red or pink and white spots (figure 2).3
The white spots on the abdomen of southern green stink bug nymphs can be used to distinguish them from green stink bug nymphs. Adults of the southern green stink bug are dull-green in color, and the fourth and fifth antennal segments are darker and reddish compared with the basal three segments (figure 3). Adults of the southern green and green stink bug can be differentiated by the presence or absence of a pointed “spine” between the base of the back legs pointed towards the head. The southern green stink bug will not have this pointed spine (figure 4). Full development from egg to adult takes between thirty and forty days, dependent on temperature.3,5 Adults of the southern green stink bug overwinter most commonly in leaf litter, under bark in wooded areas, and in structures.6

Figure 4. Underside of southern green stink bug adult (left) and green stink bug (right) with black arrows pointing to the sharply-pointed process or spine, which splits the base of the hind legs in green stink bugs and is absent in southern green stink bugs. Image credit: Scott Bundy, New Mexico State University.
Green Stink Bug, Chinavia hilaris

Figure 5. Green stink bug first instars recently hatched. Note the orangish head/thorax and black and white abdomen. Image credit: Jeremy Greene, Clemson University.
Eggs of the green stink bug are similar in color and shape to brown and southern green stink bug eggs, but they occasionally appear light green in color. Prior to hatching, the top surface of the egg turns pinkish and then red in color.7 Egg masses generally consist of between forty and fifty eggs.8 The incubation period for eggs ranges from seven to nine days.8 First instars have a reddish-orange head capsule, and the abdomen has a distinct black and white stripe pattern (figure 5). The legs of first instars are the same reddish color as the head capsule. Similar to the other species, the last antennal segments are darker in color than the first segments relative to their attachment to the head. The legs and head capsules of second and third instars lose the reddish orange color and become entirely black (figure 6). Adult green stink bugs are very similar in appearance to southern green stink bugs but are slightly larger, have dark bands on the distal ends of antennal segments (figure 7), and have the pointed abdominal spine (figure 8) described above. Development from egg to adult typically takes around thirty to forty-five days.
Brown Stink Bug, Euschistus servus
Eggs of the brown stink bug are similar in shape and color to the southern green stink bug but are laid in smaller clusters of between forty or more eggs.9 Several pinkish spots develop on the upper surface of each egg when they are close to hatching. The incubation period for brown stink bug eggs is also generally shorter than for southern green stink bug eggs, ranging from three to fourteen days.9,10 First instars of the brown stink bug have a black head capsule and light brown or tan abdomen with several black spots located centrally on the abdomen (figure 8). Second and third instars are primarily brownish tan in color and have a series of black spots on the head, pronotum, and abdomen. Fourth and fifth instars are pale green in color, have a large brown patch in the middle of the abdomen (figure 9), and reddish antennae with darker red or black coloration towards the distal segments.10 Adults of the brown stink bug are around a half inch in length and are light brown in color (figure 10). Similar to the southern green stink bug, the fourth and fifth antennal segments of the brown stink bug are darker compared with the basal segments. The underside of brown stink bug adults varies in color depending on the physiological state of the insect, with colors ranging from reddish-brown to yellow or light to dark green.11 The spined soldier bug, Podisus maculiventris, is a beneficial stink bug that can be distinguished from brown stink bugs by their sharply pointed “shoulders” and much thicker mouthparts relative to the brown stink bug (figures 10 and 11)
Full development from egg to adult averages around 44 days under laboratory conditions and is dependent on temperature.4,9 There is some conflicting evidence on the overwintering preference of brown stink bugs, which has been reported for open sites with crop residue, leaf litter, or weedy hosts, as well as for woody overwintering sites.6,12

Figure 10. Brown stink bug adult (left) and spined soldier bug (right). Note the absence of striped antennae or pattern on the margin of the abdomen and absence of sharply pointed shoulders to differentiate the brown stink bug from the brown marmorated stink bug or spined soldier bug. Note the spined shoulders on the spined soldier bug (right). Image credit: Jeremy Greene, Clemson University (left) and Rebekah D. Wallace, University of Georgia, Bugwood.org (right).
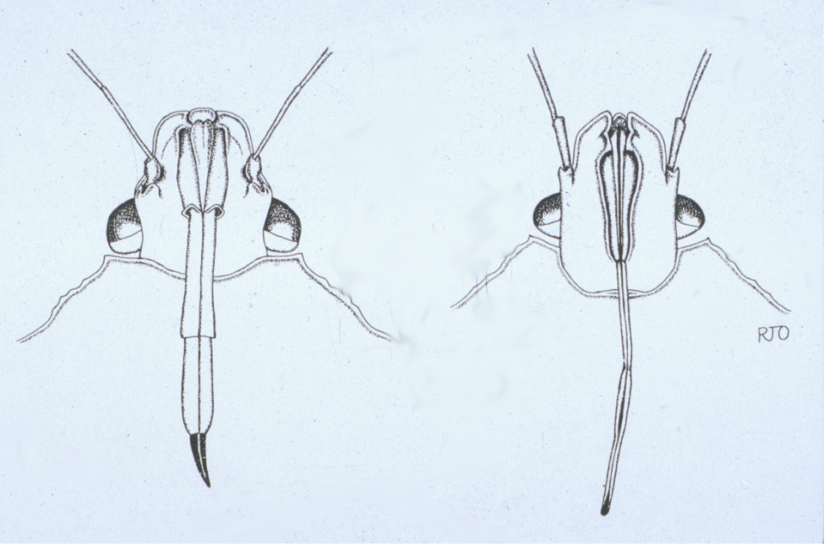
Figure 11. The difference in mouthparts between the spined soldier bug (left) and brown stink bug (right).
Image credit: Russ Ottens, University of Georgia.
Brown Marmorated Stink Bug, Halyomorpha halys

Figure 12. Brown marmorated stink bug nymphs. Image credit: Herb Pilcher, USDA Agricultural Research Service, Bugwood.org.
Eggs of the brown marmorated stink bug are laid in clusters and similar in shape and size to other stink bug species; however, the eggs are initially light green in color when deposited and turn white prior to hatching. Eggs are laid in clusters of around thirty eggs and take three to six days to hatch.13 First instars have a black head and a reddish orange abdomen with black markings similar to other species (figure 12). The color of the abdomen becomes more pale orange in color as it develops to the second instar. Third through fifth instars are primarily dark brown in color with several orange markings on the abdomen and thorax (figure 13). The adult brown marmorated stink bug most closely resembles the native brown stink bug but can be distinguished by lighter banding present both on the antennae and on the margins of the abdomen (figure 14).14 Full development from egg to adult takes between thirty-two and thirty-five days at an average temperature of 30°C. The brown marmorated stink bug is also known to be a significant nuisance pest, invading homes and structures to overwinter, which is relatively unique amongst the pentatomids described here. In addition, brown marmorated stink bugs can overwinter in wooded areas, similar to the other stink bugs.14
Damage to Cotton
Feeding by stink bugs on seedling cotton does not have an impact on the growth potential of plants, and feeding on flowers or squares (i.e., flower buds) also has limited injury potential.15 Feeding directly on bolls by adults and nymphs is the primary mechanism for economic injury from stink bugs. Boll feeding results in the shedding of newly formed bolls, carpel injury (warts) (figure 15), reduced overall boll growth, seed cotton yield, stained lint, an increase in the percentage of hardlocked bolls (figure 16),15,16 and introduction of fungal and bacterial contamination17. Hardlock occurs when bolls develop normally and perhaps partially open, but the fiber does not dry out and “fluff up” as normal.18 Hardlock bolls cannot be harvested and result in yield loss. The causal agent of hardlock bolls is generally microbial contamination, which is facilitated by stink bug feeding and not a direct result of the stink bug feeding itself. The easiest symptom of injury to identify during the season, which can aid in determining stink bug population levels in the field, are callus growths, referred to as warts, on the inside of boll walls (figure 15).17,18
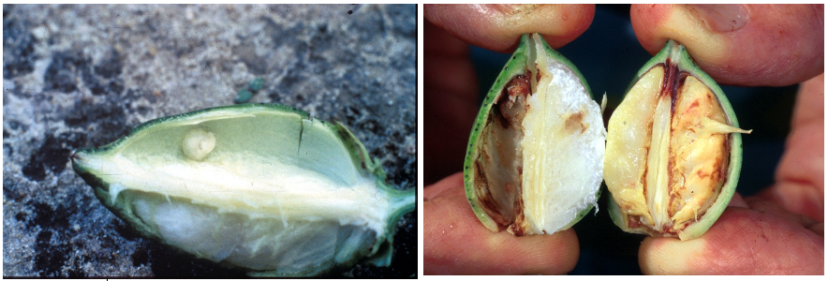
Figure 15. Wart in stink bug injured boll (left) and severely injured boll (right). Image credit: Jeremy Greene, Clemson University.

Figure 16. Stink bug injured bolls as a result of stink bug feeding. Image credit: Jeremy Greene, Clemson University.
Management
Stink Bug Sampling and Thresholds
The most reliable means of sampling stink bugs for treatment decisions is opening bolls to look for injury. When scouting, a minimum of twenty-five bolls should be selected from at least several different areas of the field, opened by hand, and examined for symptoms of stink bug feeding injury (i.e., warts and stained lint). Targeted bolls should be the largest, softest (easily mashed between thumb and finger) bolls available, and these are roughly the diameter of a $0.25 coin (USD) or a little larger. For fields larger than twenty-five acres, add one additional boll sample for each acre (25 bolls + 1 additional for each acre over twenty-five acres). The action threshold for stink bug injury varies based on the week of bloom. Starting at the first week of bloom, the action threshold for South Carolina is 50, 30, 10, 10, 10, 20, 30, and 50% percent of quarter-sized bolls that are damaged.18 Weeks three through five of bloom are the most critical for monitoring stink bug damage because the highest proportion of bolls susceptible to stink bugs are available during that span. Therefore, the action threshold is the lowest (10%) at those stages.
When an insecticide treatment is necessary based on boll sampling, populations of stink bugs should be sampled to determine the species composition in a given field. Because the brown stink bug is more tolerant of most pyrethroid insecticides than other stink bug species, the use of bifenthrin is recommended, or, alternatively, an organophosphate (acephate or dicrotophos) can be tank-mixed with other pyrethroids to achieve good control when brown stink bug is the dominant species. The two primary tools for sampling stink bugs in cotton are the drop cloth (i.e., beat sheet or ground/beat cloth) and the sweep net. Sweep nets can be purchased from a number of different retailers, and drop cloths can be purchased or easily made by hand with a 3-by-3-foot piece of heavy white or black cloth and some wooden dowels. Sweep-net sampling can be more effective at low stink bug densities, but using a drop cloth will give a better estimate of reproduction by detecting nymphs.1 To sample for stink bugs in cotton, several different areas of the field should be selected, with a particular focus on the edges of fields, especially those bordering peanuts, corn, or soybeans. In addition to determining species composition, if boll sampling is not conducted, an alternative action threshold based on drop-cloth sampling of one stink bug per six feet of row can be used.2 Solely using the threshold based on stink bug density is not recommended, as sampling boll injury generally provides a more accurate assessment of stink bug infestations compared to highly mobile stink bugs, which are more difficult to sample. Stink bugs typically invade fields starting at the field margin and can aggregate there.1 Particular focus should be placed on scouting at the edge of the field and then sampling more interior sections if stink bugs are found on field margins.
Biological Control
Egg predators and parasitoids naturally occur in the field and can be beneficial in limiting the populations of stink bugs. The parasitoid, Trichopoda pennipes, deposits eggs on the outer surface of adults (figure 7), resulting in the eventual death of the stink bug host. In particular, cotton that is in close proximity to corn can have a higher rate of egg parasitism, as these biological control agents will move between crops with stink bugs.19 Applying insecticides only when stink bug populations reach the economic injury level in cotton can help to preserve biological control agents.
Cultural Control
Stink bugs are highly polyphagous pests, and many of their crop hosts are often planted in close proximity to each other in the southeastern United States. Many of these hosts function as preferred hosts for stink bugs at different points in the season, correlated mostly with the availability of fruiting structures.20 The density and growth stage of hosts around cotton fields can play a role in the infestation levels of stink bugs in cotton. Corn is a preferred early to mid-season host for stink bugs, so scouting population levels in corn near cotton fields may provide some insight into potential movement into cotton.21 Later in the season, soybean and peanut are preferred hosts for stink bugs, and cotton planted adjacent to these crops is often at a greater risk of significant stink bug damage.20, 21
Trap cropping involves the planting of an attractive plant host around the crop, which acts as a sink for the pest species to keep them away from the crop. This cultural practice has been investigated as a potential management tactic for stink bugs in cotton. Planting of grain sorghum along an interface between corn and cotton helped to reduce populations of southern green stink bugs in cotton.22 Pheromone traps combined with a physical barrier made with thick plastic sheets have also been evaluated as tactics to prevent movement of stink bugs into cotton.23 However, more research is needed to further evaluate and optimize such approaches before they can be recommended to manage stink bugs in cotton.
Chemical Control
There are several options for insecticide modes of action that can be effective for managing stink bugs in cotton once they reach damaging levels. Pyrethroids, organophosphates, and insect growth regulators (i.e., novaluron) can all be effective for managing stink bugs. Insect growth regulators are generally more environmentally friendly and compatible with biological control, but they are only effective when populations are primarily composed of nymphs, so scouting is important. When the primary species in the field is the brown stink bug, bifenthrin or dicrotophos will be more effective than any of the other pyrethroid or organophosphate insecticides. High rates of pyrethroid insecticides are often needed for and provide good initial and residual control of stink bugs. To manage the development of insecticide resistance, if multiple applications are needed in the same season for stink bugs, the insecticide mode of action should be rotated. For example, if an organophosphate application is made, the next insecticide application should be a pyrethroid or another mode of action. Updated recommendations on using insecticides in cotton are provided in the South Carolina Pest Management Handbook (clemson.edu/extension/agronomy/pest-management-handbook.html).
Conclusions
This publication provides an overview of stink bugs as pests of cotton. An integrated pest management approach should be used to manage these pests, with chemical control used as a last resort.
References
- Reay-Jones FPF, Greene JK, Toews MD, Reeves RB. Sampling stink bugs (Hemiptera: Pentatomidae) for population estimation and pest management in southeastern cotton production. Journal of Economic Entomology. 2009;102(6):2360–2370.
- Greene JK, Turnipseed SG, Sullivan MJ, May OL. Treatment thresholds for stink bugs (Hemiptera: Pentatomidae) in cotton. Journal of Economic Entomology. 2001;94(2):403–409. doi:10.1603/0022-0493-94.2.403
- Todd JW. Ecology and behavior of Nezara viridula. Annual review of entomology. Vol. 34. 1989:273–292. doi:10.1146/annurev.ento.34.1.273
- Munyaneza J, McPherson JE. Comparative study of life histories, laboratory rearing, and immature stages of Euschistus servus and Euschistus variolarius (Hemiptera: Pentatomidae). Great Lakes Entomologist. 1994;26(4):263–274.
- McPherson RM, McPherson JE. Stink Bugs of economic importance in America north of Mexico. Boca Raton (FL): CRC Press; 2000. 253 p.
- Jones WA, Sullivan MJ. Overwintering habitats, spring emergence patterns, and winter mortality of some South Carolina Hemiptera. Environmental Entomology. 1981;10(3):409–414. doi:10.1093/ee/10.3.409
- Whitmarsh RD. The green soldier bug. Wooster (OH): Ohio Agricultural Experiment Station; 1917. Bulletin
- Whitmarsh RD. The green soldier bug (Nezara hilaris). Journal of Economic Entomology. 1914;7(4):336–339. doi:10.1093/jee/7.4.336
- Rolston LH, Kendrick RL. Biology of the brown stink bug, Euschistus servus Say. Journal of the Kansas Entomolgical Society. 1961;34(3):151–157.
- Munyaneza J, McPherson JE, Koppel AL, Herbert DA, Kuhar TP, Kamminga K. Comparative study of life histories, laboratory rearing, and immature stages of Euschistus servus and Euschistus variolarius (Hemiptera: Pentatomidae). Environmental Entomology. 1994;38(2):263–274. doi:10.1603/022.038.0209
- Babu A, Del Pozo-Valdivia AI, Reisig DD. Baseline flight potential of Euschistus servus (Hemiptera: Pentatomidae) and its implications on local dispersal. Environmental Entomology. 2020;49(3):699–708. doi:10.1093/ee/nvaa041
- Babu A, Reisig DD, Walgenbach JF, Heiniger RW, Everman W. Influence of weed manipulation in field borders on brown stink bug (Hemiptera: Pentatomidae) densities and damage in field corn. Environmental Entomology. 2019;48(2):444–453. doi:10.1093/ee/nvz016
- Kawada H, Kitamura C, Kawada H, Kitamura C. The reproductive behavior of the brown marmorated stink bug, Halyomorpha mista Uhler (Heteroptera: Pentatomidae) I. Observation of mating behavior and multiple copulation. Applied Entomology and Zoology. 1983;18(2):234–242. doi:10.1303/aez.18.234
- Rice KB, Bergh CJ, Bergmann EJ, Biddinger DJ, Dieckhoff C, Dively G, Fraser H, Gariepy T, Hamilton G, Haye T, et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). Journal of Integrated Pest Management. 2014;5(3):1–13. doi:10.1603/ipm14002
- Willrich MM, Leonard BR, Temple J. Injury to preflowering and flowering cotton by brown stink bug and southern green stink bug. Journal of Economic Entomology. 2004;97(3):924–933. doi:10.1093/jee/97.3.924
- Wene GP, Sheets LW. Notes on and the control of stink bugs affecting cotton in Arizona. Journal of Economic Entomology. 1964;57(1):60–62.
- Medrano EG, Bell AA, Greene JK, Roberts PM, Bacheler JS, Marois JJ, Wright DL, Esquivel JF, Nichols RL, Duke S. Relationship between piercing-sucking insect control and internal lint and seed rot in southeastern cotton (Gossypium hirsutum L.). Journal of Economic Entomology. 2015;108(4):1540–1544. doi:10.1093/jee/tov156
- Greene JK, Turnipseed SG, Sullivan MJ, Herzog GA. Boll damage by southern green stink bug (Hemiptera: Pentatomidae) and tarnished plant bug (Hemiptera: Miridae) caged on transgenic Bacillus thuringiensis cotton. Journal of Economic Entomology. 1999;92(4):941–944. doi:10.1093/jee/92.4.941
- Tillman PG. Natural biological control of stink bug (Heteroptera: Pentatomidae) eggs in corn, peanut, and cotton farmscapes in Georgia. Environmental Entomology. 2011;40(2):303–314. doi:10.1603/EN10154
- Grabarczyk EE, Mizell RF, Greene JK, Herzog GA, Tillman PG, Cottrell TE. Spatiotemporal distribution of two Euschistus spp. stink bugs (Hemiptera: Pentatomidae) in southeastern farmscapes. Journal of Insect Science. 2021;22(1):1–12. doi:10.1093/jisesa/ieab111
- Toews MD, Shurley DW. Crop juxtaposition affects cotton fiber quality in Georgia farmscapes. Journal of Economic Entomology. 2009 Aug; 102(4):1515-1522.
- Tillman PG. Sorghum as a trap crop for Nezara viridula L. (Heteroptera: Pentatomidae) in cotton in the southern United States. Environmental Entomology. 2006 Jun;35(3):771–783.
- Tillman PG, Khrimian A, Cottrell TE, Lou X, Mizell III RF, Johnson CJ. Trap cropping systems and a physical barrier for suppression of stink bugs (Hemiptera: Pentatomidae) in cotton. Journal of Economic Entomology. 2015 Oct;108(5):2324–2334.










