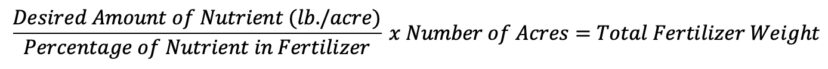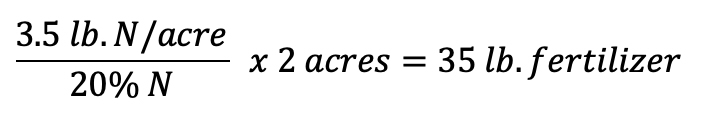Depending upon the physio-chemical properties of the fertilizer solution, many fertilizers, both solid and liquid, are suitable for fertigation (application of fertilizer dissolved in irrigation water).1 Fertigation can be a great way to improve the fertilizer use efficiency of crops, as it increases the precision by which nutrients are delivered and decreases the amount of fertilizer that goes to waste, potentially saving money. However, some fertilizer materials are incompatible in concentrated solutions, as they can react with minerals in irrigation water in tank mixes and form insoluble compounds and precipitates.2 Precipitation of fertilizers renders them unavailable for plant uptake and can further clog the micro-irrigation system. This publication focuses on fertilizers, fertilizer formulations, fertilizer compatibility, and criteria for selecting fertilizer mixtures for fertigation. This information will be helpful for growers utilizing fertigation to supply nutrients to row crops, vegetable and fruit crops, nursery and greenhouse crops, and home gardens.
Introduction
To survive, plants need sixteen essential nutrients. The most abundant elements in plants are carbon, hydrogen, and oxygen, which are derived from the atmosphere and soil. Supplemental fertilizers containing these are not needed.3 The remaining thirteen essential nutrients are acquired by plants from soil and fertilizers and are divided into two categories: macronutrients and micronutrients. Macronutrients are needed in relatively large quantities for plant survival and are further subdivided into primary (nitrogen, phosphorus, and potassium) and secondary macro-nutrients (calcium, sulfur, and magnesium).3 Micronutrients are needed in very small quantities and include iron, boron, copper, manganese, molybdenum, chlorine, and zinc. In situations where soils are deficient in one or more of these essential nutrients, fertilizers are required to meet plant demand.3
Fertilizer Formulations
Dry or liquid fertilizers can be applied in irrigation water. Liquid fertilizers may contain multiple nutrient or single nutrient materials.4 Liquid fertilizers are either clear liquids with fertilizers fully dissolved (solutions) or liquids that contain fertilizers in the form of suspended solids (suspensions). Liquid fertilizers (true solutions, not suspensions) offer several advantages over solids. Although liquid formulations are more expensive because of transportation costs, liquid fertilizers save time and labor and help prevent problems associated with poorly made stock solutions from dry, granular fertilizers.4 Granular fertilizers are dry, solid fertilizers manufactured with a special conditioner coating to keep the moisture from being absorbed by the fertilizer pellets. If dry fertilizers containing non-dissolving filler or coating materials are used, allow for a settling period (six to eight hours) within the tank before injection so sediments can settle to the tank bottom.5 Some solid fertilizers are very water soluble and are developed for fertigation, and thus are often the simplest and most efficient form of fertilizer to purchase, as they tend to weigh less than liquid solutions.
Fertilizer labels usually contain information about different sources the fertilizer is derived from (figure 1). Whenever possible, the “solution grade” form of these products should be purchased to avoid having to deal with conditioners and potential clogging problems. Some fertilizer labels specify “water soluble” or “solution grade”, while others do not (figure 1). It is vital to ensure that fertilizers used for fertigation are water soluble or solution grade. If the information is not on the product label, visit the fertilizer manufacturer’s website for product information or contact a company representative from the company manufacturing or supplying the fertilizer.

Figure 1. Examples of fertilizer bag labels listing fertilizer grade, nutrient content, and primary fertilizer materials used to derive the compound fertilizer. Image credit: Bhupinder Singh Jatana, Clemson University.
Fertilizer Compatibility
Identify Fertilizer Compatibility
Fertilizers that are water soluble are required for fertigation to minimize potential clogging problems. Dry fertilizer materials differ widely in water solubility, with solubility depending on the physical properties of the fertilizer as well as irrigation water temperature and pH.6 A popular method to deliver fertilizers more efficiently to crops is to mix multiple different fertilizer types together to provide a complete nutrient suite that may not have been available in only one fertilizer source. However, multiple problems can arise from mixing fertilizers if precautions are not taken to ensure the fertilizers are compatible with each other. While many fertilizers can be mixed and spread with no issue, some fertilizers can react with each other or not mix at all, which can either ruin the fertilizer or cause problems in irrigation and spraying systems. Figure 2 is a fertilizer compatibility chart showing the compatibility between different fertilizers when mixed. The chart can be read by finding two fertilizers of interest and finding the intersection between them. The different colors in each box of the chart represent their compatibility, with green representing fully compatible fertilizers that can be mixed and used, yellow representing limited compatibility, purple representing very limited, and red representing fully incompatible fertilizers.
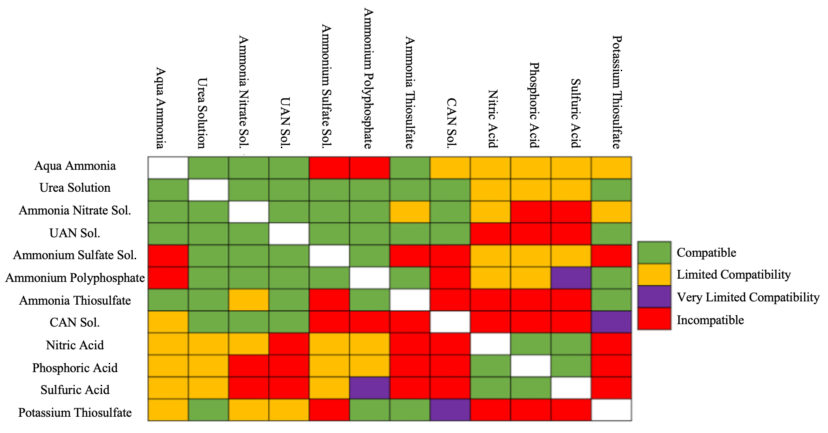
Figure 2. In a liquid fertilizer mixing compatibility chart, the color of the intersection of columns and rows identifies the fertilizer compatibility, meaning whether fertilizers can be mixed with each other or not.7 For example, aqua ammonia is compatible with ammonium nitrate solution but incompatible with ammonium sulfate solution. (Calcium Ammonium Nitrate [CAN], Urea Ammonium Nitrate [UAN]. Image credit: Bhupinder Singh Jatana, Clemson University (select the image to view a higher resolution version).
Water Chemistry and Fertilizer Compatibility
Water quality is the measure of the characteristics of a water source. Some of the criteria for irrigation water quality include salinity hazard, sodium hazard, pH, alkalinity, and specific ions.3 While water is often thought to be pure, many water sources may contain small amounts of minerals since water with no dissolved minerals does not occur naturally.8 These minerals can potentially interact with the fertilizer being pumped through a fertigation line and cause precipitates or crystals to form, reducing even distribution of liquid fertilizers through an irrigation system. For example, injecting aqueous ammonia can increase water pH enough so that salts dissolved in the water may precipitate, forming solid crystals that can clog irrigation lines and emitters.1 High bicarbonate (HCO3–) in water can cause the precipitation of calcium and magnesium in some fertilizers, such as calcium nitrate or calcium ammonium nitrate. Phosphate fertilizers are especially sensitive to precipitation when irrigation water has high levels of dissolved calcium and magnesium, especially when the irrigation water has a pH ≥ 7.5.1 Fertilizers containing sulfates can form gypsum or Epsom salt precipitates in water with a pH ≥ 7.5, and high dissolved calcium and magnesium.1 Potassium fertilizers (except sulfate of potash/potassium sulfate) generally do not cause any precipitates to form in irrigation water. A jar test can be done before fertigation to test for incompatibilities as per the method illustrated in figure 3.2
Perform a Jar Test to Evaluate Compatibility
To ensure water quality and fertilizer/fertilizer mix compatibility, perform a jar test before adding fertilizer to the supply tank (figure 3). Take a clean jar and fill it with the irrigation water in which you want to dissolve the fertilizer. Add a small amount of the chemical fertilizer to be injected so that the concentration is slightly higher than anticipated for injection, then shake well. Leave the jar overnight to permit the occurrence of any potential chemical reactions. If any cloudiness, precipitation (precipitates), or solids are observed, it is likely that clogging will occur with the chosen combination of water and chemical fertilizers.

Figure 3. The jar test steps to determine the fertilizer compatibility and unpredicted chemical reactions with irrigation water minerals, water treatments, etc. Image credit: Adapted from W. Garrett Owen, University of Kentucky, Jar Test Steps.
Criteria to Select Fertilizers for Fertigation
Soil Test
Have the soil tested several months before planting so that liming requirements can be addressed if needed. A soil test will also assess levels of available phosphorus, potassium, calcium, magnesium, and micronutrients (figure 4). Typical recommendations in soil fertility reports are expressed in elemental or oxide forms needed per planted acre. For more information on soil test reports, refer to the Land-Grant Press publication “Interpreting Routine Soil Tests”. The Agricultural Service Laboratory at Clemson University and other private laboratories can provide general recommendations for crops based on soil testing.

Figure 4. A standard soil fertility test report provided by the Clemson University Agricultural Service Laboratory. Image credit: Bhupinder Singh Jatana, Clemson University.
Calculate Crop Nutrient Requirements in Planted Acres
The recommended fertilizer requirement of most vegetable crops for South Carolina has been determined by various researchers as pounds per acre of N, P2O5 and K2O during different crop growth stages and is specified in the “Southeastern U.S. Vegetable Crop Handbook”. Another good source of information for fertilizer management in vegetable crops is the “Vegetable Production Handbook of Florida”. Use the handbooks for crop-specific nutrient requirements, as each crop has different requirements. The entire crop demand for phosphorus and micronutrients should be applied preplant.9 The total nitrogen and potassium required should be split and applied preplant (in a granular form) and injected with irrigation water in small increments during important crop growth stages.
Planted field surface area in acres = (Distance between center of one bed to center of next bed in feet × length of bed in feet × total number of beds) ÷ 43,560 sq. ft.
Use the cumulative fertilizers N, P2O5, and K2O required to calculate the total fertilizer requirement during the season.
Example
- Assume that watermelons need 150 lb. of N/acre and 150 lb. K2O/acre. A typical strategy, based on soil test results, might be to supply 35 lb. N and 35 lb. K2O/acre as preplant, and the remaining 115 lb. N and 115 lb. K2O is injected over the growing season (as per the “Southeastern U.S. 2024 Vegetable Crop Handbook”).
- Assume that the remaining N and K2O will be injected at a rate of 1 lb./acre/day and that the grower injects fertilizer once every week.
Calculations
Per day fertilizer injected = 1 lb./acre N and K2O.
If fertilizer is injected once every week, then fertilizer for 7 days (in a week) will be injected at once.
Fertilizer needed for injecting per week, per acre = 1 lb./acre/day × 7 days/week × 1 acre = 7 lb. N/week and 7 lb. K2O/week.
Assume the grower planted 2 acres:
- Amount of fertilizer needed for 2 acres = fertilizer needed for injecting 1 acre in a week × number of acres.
- Amount of fertilizer needed for 2 acres = 7 lb. N/week and 7 lb. K2O/week × 2 acres = 14 lb. N/week and 14 lb. K2O/week.
- If the grower wanted to fertigate twice a week, the amount of fertilizer needed per fertigation, per acre = 7 lb./acre/week ÷ 2 fertigation events/week = 3.5 lb. N/acre/fertigation event, and similarly 3.5 lb. K2O/acre/fertigation event.
Determine Water Volume Needed to Solubilize Dry Fertilizers
Fertigating small acreage does not require much water to solubilize the calculated amount of greenhouse-grade water-soluble dry fertilizers (needed to supply the desired amount of nutrients). Fertilizers contain impurities and other fillers, which can lower the amount of fertilizer that can be solubilized in a unit of water. For the solubility limit of the fertilizer in question, refer to the fertilizer label (figure 5). If the solubility limit is not specified on a fertilizer label, visit the fertilizer manufacturer’s website for product information or contact a representative from the company manufacturing or supplying the fertilizer. Keep in mind that the volume of the fertilizer solution to be injected should be sufficient enough to be evenly distributed throughout the system and reach the end of the system.
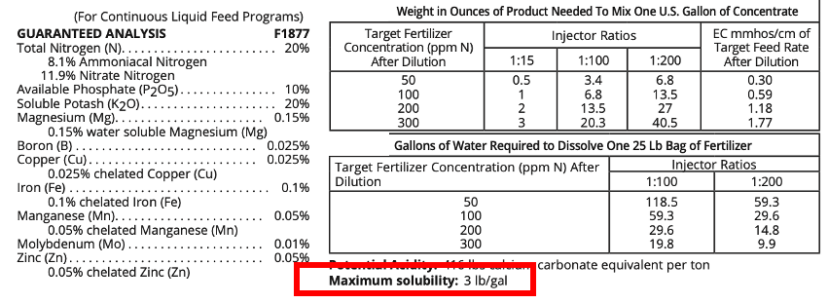
Figure 5. Water solubility limits are usually included on the fertilizer’s label. If this information is not included on the label, look up the product on the manufacturer’s website or contact a representative from the company that manufactures or supplies the fertilizer. Image credit: Screenshot by author, fertilizer label 20-10-20.
The water temperature used to solubilize the fertilizer can impact fertilizer solubility, generally resulting in larger quantities of fertilizer solubilized in warm/hot water than in cold water. To keep the fertilizer injection time as short as possible, it is convenient to use warm/hot water to reduce the total volume of fertilizer that is needed to be injected (figure 5). How temperature impacts solubility is product specific.6 After solubilizing fertilizer at higher temperatures, allow the solution to cool before injecting. It is crucial that the final fertilizer concentration is below the maximum solubility limit for the irrigation water temperature before injecting. Otherwise, any excess fertilizer beyond the solubility limit at the lower temperature can precipitate out of the solution and cause clogging issues. To calculate the amount of water needed to stabilize the desired quantity of fertilizers, refer to the fertilizer label for the solubility in water and how temperature may impact solubility. If mixing at a high temperature is followed by cooling before injection, be sure that the concentration of fertilizer in the final solution is below the maximum solubility rate for that temperature of irrigation water; otherwise, it is possible leftover fertilizer can precipitate if the concentration is above the maximum solubility for irrigation water temperature.
Example
Using the example above and the labels in figure 5, calculate the amount of fertilizer solution needed for each fertigation event to apply 3.5 lb. N/acre for a 2-acre field. Since the fertilizer is grade 20-10-20, that means that 20% of the weight of the fertilizer is nitrogen.
In order to calculate the correct amount of fertilizer to add to reach the desired amount, the following formula can be used
To find how much 20-10-20 fertilizer is needed to reach 3.5 lb./acre, the following formula can be used
The solubility of the fertilizer, as shown on the label, is a maximum of 3 lb. fertilizer per gallon of water. To find the amount of water required to solubilize all of the fertilizer, use the following calculation
35 lb. fertilizer ÷ 3 lb. fertilizer/gal. = 11.66 gal.
The minimum required amount of water to solubilize 35 lbs. of fertilizer is 11.66 gal.
Determine the Time Needed to Inject Fertilizer through an Irrigation System
Always check the fertilizer label and perform a jar test to check for incompatibilities before injecting fertilizers (figure 2).2,9 Calculations can be done by timing an actual injection of a small known volume of fertilizer mixture and calculating for a known volume of solution to be injected.6 The addition of a dye into the fertilizer solution can help you determine when the solution has reached the end of an irrigation line and spread throughout the zone as desired.9 After injecting the fertilizer, continue to run water through the line to evenly distribute all the fertilizer throughout the zone. However, any dyes used should be safe for plants, animals, and the environment. The length of this run is generally a little longer than the time required initially to bring the system up to full pressure. If needed, flush the system after a fertigation event with clean water to clear any particles from the drip tape that may clog emitters. In summary, the total time for an entire fertigation event includes the following
- Time for water to travel from the injection point to the farthest emitter to bring the system up to full pressure.
- Time to inject the fertilizer solution.
- Time for fertilizer solution to reach the farthest emitter after completing fertilizer injection.
- Additional time to flush the system if needed.
Example
Assume it takes five minutes for the water to travel from the injection point to the farthest emitters + for the system to reach full pressure, fifteen minutes for fertilizer injection, and ten minutes to flush the remaining fertilizer through the system. If needed, it takes five minutes to flush the system. This accounts for a total fertigation event duration of thirty minutes without flushing and thirty-five minutes with flushing.
Note: Fertilizers must be completely flushed from the system after fertigation to keep drip lines clean and prevent any emitter clogging.
Tank Preparation and Cleaning
Prior to injection, adjust the injection pump intake so it is between 8 in. to 10 in. (20 cm. to 25 cm.) above the surface of the sediments to avoid the sediments being taken up and subsequently clogging the lines and emitters. A flush valve should be located at the bottom of the tank to facilitate the removal of sediments after injection of the stock solution. Sediments can be flushed with running water, collected in a bucket or other container, and either spread into an area with growing plants or properly disposed of with other chemicals. Surface scums that may form in the tank when dissolving fertilizers that contain fillers or coatings will need to be skimmed off. Using water soluble fertilizer generally results in avoiding surface scums, saving time and labor.
How to Find a Fertilizer Supplier
For smaller fertilizer needs, purchasing individual bags of water soluble fertilizers for fertigation and following the label for solubilizing in water may be the best option. For large operations and acreage, there are companies that will mix a custom fertilizer solution (based on what nutrients are needed) and deliver the mixture in a fertilizer tank. Some companies have turn-key operations that include sampling the soil, conducting soil tests, recommending a fertilizer rate and form, and supplying the product ready to be injected.
To find suppliers for your desired fertilizer needs, an Internet search, including keywords related to the grade desired, bulk/small purchase size, and the form preferred (such as liquid or granular), should help to identify the right supplier for production needs. Always ensure that any fertilizer used for fertigation is water soluble. If the solubility limit is not stated on the product label, search for the product on the manufacturer’s website or call a representative from the company manufacturing or supplying the fertilizer. Contact the local Clemson Extension Agent or Specialist for additional assistance. County office contact information is available on the Clemson Cooperative Extension website.
References Cited
- Evans R, Wu I, Smajstrala AG. Micro irrigation systems. In: McCann P, editor. Design and operation of farm irrigation systems. 2nd ed. St. Joseph (MI): American Society of Agricultural and Biological Engineers; 2007.
- Owen W, Whipker B. Jar test: determining fertilizer solubility and compatibility. e-GRO (Electronic Grower Resources Online); 2022 Feb. e-GRO Alert. Volume 11, Number 8. https://e-gro.org/pdf/2022-11-08.pdf.
- Delgado A, Quemada M, Villalobos FJ. Fertilizers. In: Villalobos F, Fereres E, editors. Principles of agronomy for sustainable agriculture. New York (NY): Springer, Cham; 2016. doi:10.1007/978-3-319-46116-8_23.
- Uchida R. Essential nutrients for plant growth: nutrient functions and deficiency symptoms. In: Uchida RS, editor. Plant nutrient management in Hawaii’s soils: approaches for tropical and subtropical agriculture. Honolulu (HI): University of Hawaii at Manoa; 2000. 4, 31–55. https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pnm3.pdf.
- Fares A, Abbas F. Irrigation systems and nutrient sources for fertigation. Honolulu (HI): University of Hawaii at Manoa; 2009 May. Soil and Crop Management. SCM-25. https://scholarspace.manoa.hawaii.edu/server/api/core/bitstreams/7002a9af-ddee-4ca5-b8d6-35cf5053bf9b/content.
- Simonne E, Studstill D, Hochmuth R, Jones J, Starling C. On-farm demonstration of soil water movement in vegetables grown with plasticulture. Gainesville (FL): University of Florida Extension; 2005. HS1008/HS251, 3/2005”. EDIS 2005 (3). doi:10.32473/edis-hs251-2005.
- eKonomics. Liquid Fertilizer Mixing Compatibility – What You Should Know. Saskatoon, Saskatchewan (CA): Nutrien LTD; 2024. https://nutrien-ekonomics.com/news/liquid-fertilizer-mixing-compatibility-what-you-should-know/.
- Nelson D. Natural variations in the composition of groundwater. In: Proceedings of the Annual Meeting of the Groundwater Foundation, 2002 Nov 18–20; Valley River Inn (OR). Springfield (OR): Oregon Department of Human Services; 2002. p. 84–89. https://www.oregon.gov/oha/ph/HealthyEnvironments/DrinkingWater/SourceWater/Documents/gw/chem.pdf.
- Kumar S, Kumar S, Mohapatra T. Interaction between macro‐and micro-nutrients in plants. Frontiers in Plant Science. 2021 May 10;12:665583. doi:10.3389/fpls.2021.665583.
- Kafkaki U, Tarchitzky J. Fertigation: a tool for efficient fertilizer and water management. Paris (FR): International Fertilizer Industry Association (IFA), International Potash Institute (IPI); 2011 May. https://www.haifa-group.com/sites/default/files/ifa_fertigation-Kafkafi-511.pdf.
Additional Resources
Southeastern Vegetable Extension Workers Group (SEVEW). Kimble JM, editor. Southeastern U.S. 2024 vegetable crop handbook. 25th ed. Auburn (AL): Alabama Cooperative Extension System; 2024. https://www.aces.edu/wp-content/uploads/2023/02/2024_SEVEG_final_web.pdf.
Goldammer T. Fertigation in greenhouse production – criteria for selecting fertilizers for fertigation. In: Greenhouse management, a guide to operations and technology. 1st ed. Haymarket (VA): Apex Publishers; 2019 Apr (updated 2021 Jul). https://www.greenhouse-management.com/greenhouse_management/fertigation_greenhouse_production/criteria_selecting_fertilizers_fertigation.htm.
Polomski RF. Reading a fertilizer label. Clemson (SC): Clemson Cooperative Extension, Home & Garden Information Center (HGIC). HGIC 1228. https://hgic.clemson.edu/factsheet/reading-a-fertilizer-label/.
Snipes Z, Ballew J, Last R, Kirk KR. Clemson Drip Fertigation Calculator. Clemson (SC): Clemson University Cooperative Extension; 2022. https://precisionag.sites.clemson.edu/Calculators/Fertigation/Drip/.
Kirk KR, Last R, Snipes Z, Ballew J. Liquid Fertilizer Solution Calculator. Clemson (SC): Clemson University Cooperative Extension; 2021. https://precisionag.sites.clemson.edu/Calculators/Fertigation/LiquidSolution/.
Egbert K, Yost M, Sorenson B, Cardon G, Allen N, Larsen R. Fertigation facts. Farmington (UT): Utah State University Extension; 2019. AG/Fertigation/2019-01pr. https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=3090&context=extension_curall.
DeValerio J, Nistler D, Hochmuth R, Simmone E. Fertigation for vegetables: a practical guide for small fields. Sarasota (FL): University of Florida, IFAS Extension; 2019 Nov 4 (reviewed 2022 Dec 5). HS1206. https://edis.ifas.ufl.edu/publication/HS1206.
Snyder R. Fertigation: the basics of injecting fertilizer for field-grown tomatoes. Mississippi State (MS): Mississippi State University Extension; 2019. Publication 2037 (POD-08-19). https://extension.msstate.edu/sites/default/files/publications/publications/P2037_web.pdf.