The development of insecticide resistance in insects is common and widespread today. The long-term efficacy of insecticides is critical for successful and sustainable food and fiber production and public health. This article will discuss the development of resistance and the strategies used to manage it.
The first documented resistance to an insecticide was in 1914 with the San Jose scale, Comstockaspis perniciosus, to lime sulfur.1 Upon the development and widespread use of DDT and other synthetic insecticides in the late 1940s, the number of resistant insect species has continually increased. As of 2015, 550 insect species have been reported as resistant to 325 insecticides and five insecticidal traits in genetically engineered plants.2 Some important pest species in food production systems, such as the Colorado potato beetle (Leptinotarsa decimlineata ) and the two-spotted spider mite (Tetranychus urticae), are resistant to fifty-four and ninety-three different insecticidal compounds, respectively.3 The development of insecticide resistance eliminates management options and potentially reduces profitability for producers.
Resistance Development
Populations of insects have a mosaic of genetic traits that can result in susceptibility or competitive advantages in the natural world. When selective pressure is placed on a population, in this case, insecticides, individuals that are predisposed to effectively mitigate the toxic effects are the individuals that will survive and pass on their genetic material. The offspring of those resistant individuals then can have the same genes resulting in resistance. Continued exposure of subsequent generations to the same types of insecticides can result in a continual increase in the number of individuals that have the genetic advantage of resistance to that insecticide. Individuals can have several versions of each gene called alleles. The alleles responsible for resistance can be dominant, or more frequently recessive. When an individual has two different alleles at a given location, they are considered heterozygous. When an allele resulting in resistance is recessive, heterozygous individuals will not express that resistance, but the offspring of two heterozygous individuals have a higher chance of expressing resistance. Dominant resistance alleles result in a much more rapid development of resistance.
The development of resistance and the rate at which it develops are dependent on several factors, including the selection pressure placed on the target species. Selection pressure is controlled by the biology of the pest, the insecticide and its specificity, and the application strategy used. Insects that have multiple generations a year do not migrate, and have a narrow host range are most likely to become resistant, as they have the highest potential for repeated exposure across multiple generations. The rate of resistance development is also highly dependent on the type and method of insecticide application. Resistance develops more readily to pesticides that persist longer in the environment, have a single mode of action (MoA), and are used frequently in the same season. A toxin with a single target site is less of an evolutionary barrier to resistance than a toxin that has multiple effects.
Mechanisms of Resistance
Some of the most common mechanisms of insecticide resistance include enhanced metabolic processing of toxins, target site alterations, and alteration of how toxins are processed to improve sequestration or excretion of the toxin.4 There are several barriers that insects naturally employ to prevent toxins from reaching their target site or to reduce their effectiveness at that target site. Often, it is not a single mechanism responsible for resistance development but rather the influence of several factors.
Insecticide Resistance Management
Overview
Strategies of insecticide resistance management (IRM) generally fall under three guiding principles.5 The first is creating low selection pressure in combination with non-chemical control measures. The implementation of integrated management strategies that do not involve chemical control, such as biological and cultural controls, is the most effective strategy for managing resistance development. Reducing pest populations below an economic level through cultural control measures, such as resistant plant varieties, crop rotation, and mechanical control, or through the protection or introduction of biological control agents, decreases exposure to chemicals and resistance development. When chemical management is applied, selection pressure can be limited by reducing the overall number of sprays or by using a high dose of active ingredient that eliminates all heterozygous individuals.
The second principle is saturation of the insect defense mechanisms through increased uptake of insecticide or the use of synergistic compounds to overcome metabolic defenses. Combining insecticides with surfactants to increase contact surface area on insects has the potential to increase the amount of active ingredient that is absorbed or ingested and reaches the target site. Once an active ingredient enters the insect, the main barriers to reaching the target site are enzymes that detoxify insecticides. Insecticide synergists work in combination with the active materials to modify the activity of metabolic enzymes and increase the effectiveness of the insecticide. Synergists alone usually do not have any toxic effect, but, when used in combination with certain insecticides, they result in greater toxicity than if the two compounds are used separately. There are several different types of synergists that overcome the most common metabolic processes in insects. For example, piperonyl butoxide (PBO) binds to oxidase enzymes, which are the most common detoxifying agents in insects. This binding reduces the amount of active material they can process, increasing the toxicity of many insecticides. Insecticide formulations often are premixed to contain PBO to increase their toxicity. Some products for indoor and outdoor household use (e.g., foggers, sprays, and dusts), termite treatments, and some agricultural products contain PBO.6
The third principle of resistance management is using a mixture or a rotation of unrelated insecticides (different target sites) to eliminate excessive selection pressure from a single insecticide and reduce the development of resistance traits. Using a single insecticide multiple times in a single generation creates strong selection pressure towards individuals with one or more mechanisms of resistance, particularly when using chemistries that have a single target site. Rotating chemistries eliminates the individuals resistant to one chemistry and delays the development of that resistance to one or all of the insecticides used. Different insecticides target and disrupt different locations in insect physiological processes, and this target site activity has allowed the classification of insecticides into MoA groups. The classification of insecticidal MoAs to improve the effectiveness of rotation of active ingredients was created and is currently maintained by the Insecticide Resistance Action Committee (IRAC).
Insecticide Resistance Action Committee
Established in 1984, IRAC is an international association of key technical personnel from agrochemical and public health companies affiliated with crop protection. New insecticides can take around $100 million and eight to ten years to release, so IRAC was formed based on companies’ recognition of their vested interest in prolonging the efficacy of their existing products. Their mission is to prolong the efficacy of insecticides, miticides, and acaricides through effective resistance management. One of the primary ways to achieve this is by continual development of the MoA classification scheme (figure 1). The classification of insecticides seeks to inform users about insecticide MoAs to promote the use of different chemistries throughout the season, delaying the development of resistant populations. Classification is primarily based on the target site of the insecticide, and secondarily on the novelty of the chemistry of the insecticide.2 Some of the most commonly used insecticides and their classifications are found in table 1.
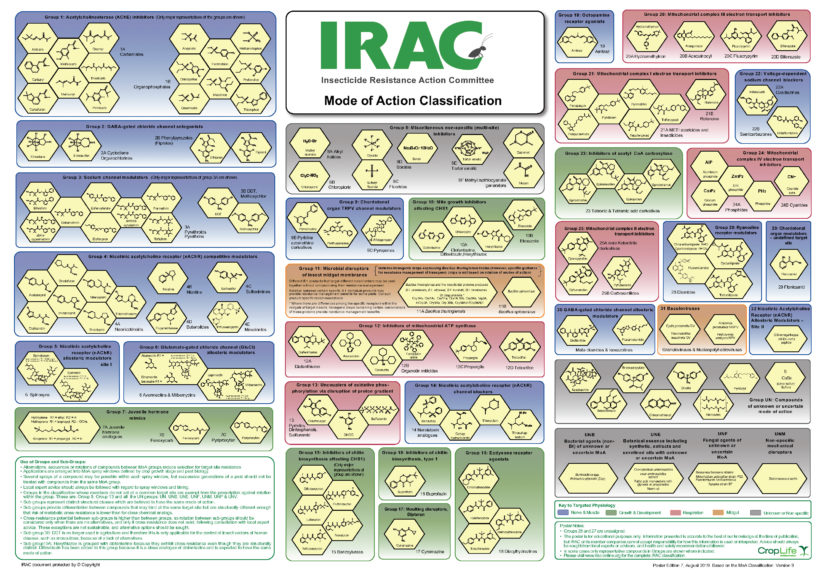
Figure 1. IRAC Mode of Action classification scheme of insecticides. Image credit: © by the Insecticide Resistance Action Committee (IRAC), www.irac-online.org.
Table 1. A selection of commonly used insecticides and their classifications.
| IRAC Group | Mode of Action (MoA) | Chemical Group | Active Ingredients |
| 1 | Acetylcholinesterase inhibitors | 1A Carbamates
1B Organophosphates |
Carbaryl,
Methomyl |
| 3 | Voltage gated sodium channel modulators | 3A pyrethroids and pyrethrins
3B DDT and analogs |
Bifenthrin,
Lambda-cyhalothrin, DDT |
| 4 | Nicotinic acetylcholine receptor agonist | 4A neonicotinoids
4B nicotine 4C Sulfoximines |
Acetamiprid,
Nicotine, Sulfoxaflor |
| 5 | Nicotinic acetylcholine receptor allosteric modulator | Spinosyns | Spinosad,
Spinetoram |
| 11 | Midgut membrane | 11A Bacillus thuringiensis |
Source: IRAC, www.irac-online.org.
Note: IRAC indicates the classification grouping number, where each number represents a unique MoA. The MoA for an IRAC group describes the target site within insects to which an active material binds. The chemical groups are the functional and structural groupings of chemistries. Sub-groups with the same mode of action may have structural differences in chemistries but are still active at the same target site. These sub-groups are denoted by a letter following the IRAC grouping number.
Resistance Management in Transgenic Technology
Much of the corn and cotton planted in the US contains genes from Bacillus thuringiensis (Bt), a bacterium that produces insect-specific toxins. These Bt crops produce one or more of these toxins that specifically target a small group of pests. There are a number of different genes expressing unique toxins that are either used singularly or in pyramided or stacked hybrids. A stacked hybrid expresses toxins for both above and below ground pests, and a pyramided hybrid expresses multiple toxins for the same pest. Each gene expresses a single toxin that has a specific target site. When insects are repeatedly exposed to the same Bt toxin, individuals that can mitigate the toxic effects are able to pass on their genetic material and increase the percentage of the population that is partially or fully resistant. Resistance to several Bt toxins has been documented in several pests.7,8
In some cases, such as with the corn earworm in corn or bollworm in cotton (Helicoverpa zea), there is potential for H. zea populations to develop resistance from selection pressure in Bt corn. These resistant individuals can then migrate to cotton later in the season, where they are again exposed to the same or similar toxins. Corn earworm resistance management is of particular concern in southeastern states where corn and cotton are both grown, and the preservation of the efficacy Bt traits is critical for sustainable production, in particular in cotton production where the insect is a major pest.
The primary strategy used to manage resistance to Bt toxins is the high-dose/refuge strategy.9 The high dose portion of this strategy involves deploying Bt crops to produce toxins at high enough levels to eliminate 95% of heterozygous resistant individuals. Refuges of plants that do not produce Bt toxins are used to generate insects that are susceptible to Bt toxins. These susceptible insects can then mate with potential resistant insects, which dilutes potential resistance genes in the population. Refuge requirements are outlined below for corn and cotton. Growers can help delay resistance by following these refuge requirements.
Non-Bt Refuge for Corn
Corn growers are required to plant a certain percentage of their corn acreage with non-Bt seed to delay the development of resistance. These refuge requirements were established by the EPA and are currently enforced by seed companies. The percentage and method of refuge planted are dependent on the growing region. In cotton-growing regions (which includes South Carolina), there is a minimum of 20% refuge required for corn expressing multiple toxins, and a refuge of 50% is required for corn expressing a single toxin. Refuge plants can be planted in blocks in a field with Bt corn, in a separate field within one-half mile of the Bt cornfield, or in strips of at least four rows in the field with Bt corn. In counties not growing cotton, non-Bt refuges can also be planted as blends of Bt and non-Bt seed. While a blended refuge is easy to implement, it is not permitted in cotton-growing counties because of the economic importance of corn earworm/bollworm as a pest of cotton, and the importance of having an effective refuge in corn. A blended refuge is considered less effective than a block refuge for ear-feeding pests because of cross-pollination from Bt plants to a refuge plant in a blend, which exposes insects to sub-lethal doses of Bt toxins, and potentially speeds up the development of resistance.10
Non-Bt Refuge for Cotton
In cotton, there is no requirement to plant a non-Bt cotton refuge in southeastern US states, because research has shown that there is enough natural refuge later in the season when bollworm is a key host of cotton. Helicoverpa zea can develop on a broad range of host plants, and the majority of individuals present later in the season originate from plants other than cotton,11 which means a non-Bt cotton refuge is not needed to provide susceptible moths. In other cotton-growing regions in the US, a structured refuge is required.
Conclusion
Numerous insects have developed resistance to insecticides. IRM practices should be implemented to delay resistance development and preserve the efficacy of insecticides as a valuable tool within integrated pest management programs. Continued efforts to preserve the effectiveness of insecticides through resistance management are critical for successful food and fiber production.
References
- Melander AL. Can Insects Become Resistant to Sprays? Journal of Economic Entomology. 1914;7(2):167–173. doi:10.1093/jee/7.2.167.
- Sparks TC, Nauen R. IRAC: Mode of action classification and insecticide resistance management. Pesticide Biochemistry and Physiology. 2015;121:122–128. doi:10.1016/j.pestbp.2014.11.014.
- Arthropod Pesticide Resistance Database. [accessed 2020 Apr 6]. https://www.pesticideresistance.org.
- Clark JM, Yamaguchi I. Scope and status of pesticide resistance. In: Agrochemical Resistance. 2001. p. 1–22. doi:10.1021/bk-2002-0808.ch001.
- Georghiou GP. Principles of insecticide resistance management. Phytoprotection. 2005;75(4):51–59. doi:10.7202/706071ar.
- Piperonyl Butoxide: Technical Fact Sheet. National Pesticide Information Center. 2000 [accessed 2020 Apr 6]. http://npic.orst.edu/factsheets/archive/pbotech.pdf.
- Bilbo TR, Reay-Jones FPF, Reisig DD, Greene JK. Susceptibility of corn earworm (Lepidoptera: Noctuidae) to Cry1A.105 and Cry2Ab2 in North and South Carolina. Journal of Economic Entomology. 2019;112(4):1845–1857. doi:10.1093/jee/toz062.
- Gassmann AJ, Petzold-Maxwell JL, Clifton EH, Dunbar MW, Hoffmann AM, Ingber DA, Keweshan RS. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(14):5141–5146. doi:10.1073/pnas.1317179111.
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annual Review of Entomology. 1998;43(1):701–726. doi:10.1146/annurev.ento.43.1.701.
- Yang F, Kerns DL, Head GP, Leonard BR, Levy R, Niu Y, Huang F. 2014. A Challenge for the seed mixture refuge strategy in Bt maize: impact of cross-pollination on an ear-feeding pest, corn earworm. PLoS ONE 9: e112962. doi:10.1371/journal.pone.0112962.
- Head G, Jackson RE, Adamczyk J, Bradley JR, Van Duyn J, Gore J, Hardee DD, Leonard BR, Luttrell R, Ruberson J, et al. Spatial and temporal variability in host use by Helicoverpa zea as measured by analyses of stable carbon isotope ratios and gossypol residues. Journal of Applied Ecology. 2010;47:583–592.

