This publication will provide turfgrass managers with the information to understand how biological control organisms work, why reliable control is not being observed, and provide strategies for increasing success when using biological control organisms. While this publication discusses biological control organisms using turfgrass diseases as examples, similar concepts apply to other pests (i.e., insects, nematodes, and weeds) and other plant systems.
Introduction
Managing plant pests with biological control organisms has been a consistent area of intrigue for scientific research, particularly in plant pest control. Interest in reducing the use of synthetic pesticides has rapidly increased among the general public. This is especially true in turfgrass, where interest in biological management of pests is rooted in a desire to reduce the exposure of pets and children to synthetic pesticides. There are numerous benefits to using biological control organisms in lieu of synthetic pesticides. Typically, restricted entry intervals are shorter (or non-existent), personal protective equipment may be reduced for applicators, non-target organisms are generally less affected, and the development of pesticide resistance is reduced.1 However, these benefits are irrelevant if biological control organisms cannot provide acceptable reductions of pest damages. Lack of control is one of the most cited concerns voiced by turfgrass managers for not adopting biological control as a management tool.
Basics of Biological Control Organisms
Understanding the inconsistencies with biological management of turfgrass pathogens first requires a basic understanding of the overarching form of suppression and the underlying modes of action that subdue pathogens. There are two forms of suppression that biological management of pathogens can take: 1) general and 2) specific suppression. General suppression involves communities of microorganisms reducing pathogen activity or disease severity2 (e.g., suppressive soils). The web of interactions that drive general suppression is currently not well understood. Conversely, specific suppression occurs through a known microorganism (e.g., commercially available biological control organisms) directly inhibiting a pathogen through one or more of four modes of action.3 These modes of action are antibiosis (i.e., production of antibiotics or inhibitory compounds), competition for space and resources (i.e., nutrients), parasitism/predation, and plant defense induction.3 Unlike synthetic fungicide active ingredients that work through a single mode of action, the lines between biological control organism modes of action may be less discrete. There are strains of Trichoderma fungi that produce antagonistic secondary metabolites and parasitize Sclerotinia sclerotiorum (a prominent fungal plant pathogen)4, and some strains of Pseudomonas bacteria produce special molecules (e.g., siderophores) that help the bacteria compete for iron in the soil5, and can also trigger plant defenses.6 Applied biological control organisms need to establish sufficient populations to reach a state where their functional ability to suppress pathogens can commence. In contrast, the active ingredients of synthetic fungicides are applied in a state where pathogen uptake is the main barrier to efficacy. Simply put, biological control organisms fundamentally do not function the same way as synthetic fungicides, and therefore, one cannot expect to implement a biological control organism the same way as a synthetic fungicide.
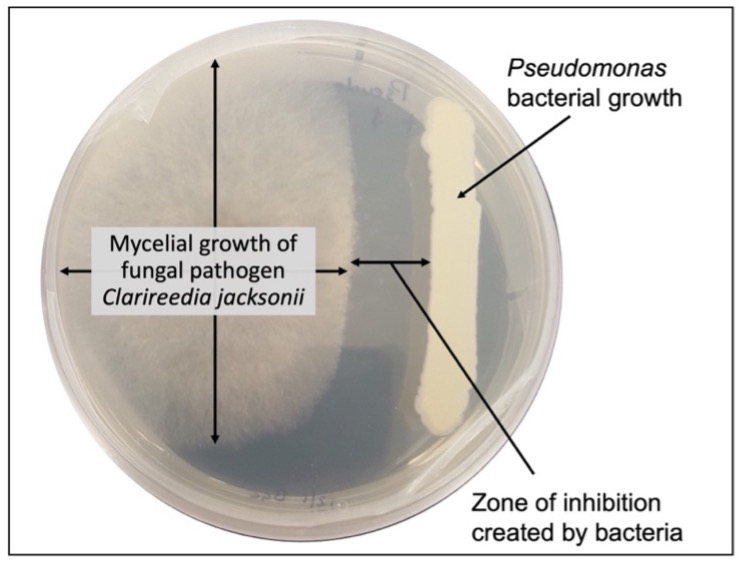
Figure 1. A Pseudomonas bacterium inhibiting the growth of the dollar spot fungus, Clarireedia jacksonii, on growth media. Image credit: Joseph Doherty, Clemson University.
The population level of an applied biological control organism influences many factors that contribute to its success. Bacteria use a mechanism called quorum sensing to regulate gene activity in response to population fluctuations.7 Quorum sensing essentially means that bacteria require a high density of cells (i.e., quorum) to initiate activities like enzyme production, pathogenicity, and sometimes movement. Regarding biological control organisms, secondary metabolites such as antibiotics and inhibitory compounds are regulated by quorum sensing.8,9 Antibiosis with a high population of bacterial cells has been easily observed in laboratory experiments, where diffusion of secondary metabolites in agar plates leads to a cessation of fungal growth before the fungus and bacteria come into contact (figure 1). If quorum sensing detects drops in populations, metabolic shifts that have negative impacts on biological control organism success can occur. In endospore (i.e., a resilient, long-term survival structure) producing bacteria, when a population decrease is detected, metabolic function will shift to producing endospores for survival.7 Switching metabolism focus to producing survival structures can reduce a biological control organism’s pathogen suppression ability as resources are diverted away from growth and secondary metabolites (e.g., antibiotics). Research is still being conducted to fully understand the complex metabolic pathways and gene expression that quorum sensing controls. Beyond secondary metabolites, population levels likely influence a biological control organisms’ ability to compete against or attack plant pathogens and induce host defenses, though additional research is needed. While there is still considerable uncertainty on precisely how biological control organisms work, increasing the reliability and efficacy of biological control organisms will rely on the ability to establish and maintain biological control organism populations before and when pathogens are active.
Challenges Facing Biological Control Organisms
Environmental Challenges
From the outset, applied biological control organisms face multiple challenges to their establishment, including turfgrass plants hosting diverse microbial communities10 that are already experiencing competition for resources. Many commercially available biological control organisms are formulated and packaged in a dormant state to increase shelf stability. Not only are biological control organisms exiting a dormant state following application, but they also immediately enter a community rife with competitors that have an advantage. A few calculations relating the discrepancy between the size of native microbial communities and applied biological control can put this into perspective. Every gram of soil harbors roughly 1 billion bacteria.11 This translates to there being about 1 quadrillion (1015) native bacterial cells in 1000 ft2 of soil. A typical application of a biological control organism introduces 1 million biological control organism cells per 1000 ft2. Meaning that there are 1 billion native soil bacteria for every 1 biological control organism cell that is applied or a 0.000000001% change. Consider, too, that there are other microorganisms in the soil besides bacteria that are competing for the same resources. This is not an insurmountable challenge though, and some would say you just need the right organism. Some of the pathogens we see in turfgrass systems can quickly colonize and overcome existing microbial populations. For instance, gray leaf spot, caused by Pyricularia grisea, starts from spores carried with the wind from the south each season, and it devastates turfgrasses year in and year out in the transition climate zones of the eastern United States. As research continues in the realm of biological control, successful products will ultimately have to possess super microbes that can overcome existing populations.
Inherent Challenges of Turfgrass Systems
The perennial nature of turfgrass systems presents some unique challenges when it comes to managing diseases. Many turfgrass pathogens grow over many months when environmental conditions are favorable. Favorable conditions for dollar spot (Clarireedia spp.) can start in spring and last through autumn months.12 Furthermore, throughout a calendar year or growing season where turfgrasses go completely dormant, some pathogens constantly assault turfgrasses. In summer months, cool-season turfgrass can be infected with dollar spot, brown patch (Rhizoctonia solani), anthracnose (Colletotrichum cereale), Pythium root rot (Pythium spp.), Pythium blight (Pythium spp.), yellow spot (algae), copper spot (Gleocercospora sorghi) and summer patch (Magnaportheopsis poae), while warm season turfgrasses can be infected with dollar spot, mini ring (Rhizoctonia zeae), leaf spot (Bipolaris spp.), ink spot (Curvularia malina), Pythium root rot, Pythium blight, and take all root rot (Gaeumannomyces spp.).12 In Indiana, for example, there is not a single month where there is no pressure from pathogens, and in July, conditions are favorable for seven pathogens.13
Plant diseases are classified as either polycyclic or monocyclic, depending on how many infection cycles occur per crop cycle. In other words, pathogens that cause polycyclic diseases infect the host, colonize, produce new inoculum, and disperse said inoculum, and new infections occur multiple times per crop cycle.14 Polycyclic diseases can have exponential increases in inoculum load in a growing season, making managing these diseases difficult, even with synthetic fungicides. Many of the highly problematic foliar diseases (dollar spot, brown patch, gray leaf spot, anthracnose) of turfgrasses are polycyclic. Making matters worse, these diseases have overlapping favorable environmental conditions.
Beyond environmental challenges, the adoption of biological control organisms is hindered by several “self-imposed” challenges. In high-value areas (i.e., golf courses or athletic fields), where disease control measures are frequently used, there is very little, if any, acceptable level of disease damage. A threshold of near zero can be tough to achieve with a strong integrated pest management program that includes conscientious use of the best synthetic fungicides. Replacing synthetic fungicides with the unpredictability of biological control organisms exponentially increases the challenge of maintaining that near zero level of disease damage. While this threshold is “self-imposed” by players, managers, and those who love to push the limits of what turfgrass managers can achieve, it is still an important consideration and significant challenge facing the adoption of biological control organisms.
Inferences from Current Research
So far, it may seem like implementing biological control organisms for managing turfgrass pathogens is a lost cause. The odds certainly seem stacked in the pathogens’ favor, and history has not presented the best case either. A review of 24 Plant Disease Management Reports that tested biological control organisms against turfgrass pathogens in field trials found that disease severity was reduced by biological control organisms in just 33% of trials.15 Since this review, researchers have continued evaluating biological control organisms, and valuable information continues to be found.
Recent research efforts have determined that there are completely different population dynamics for foliar and root zone applied biological control organisms. Foliar populations of the biological control organism Bacillus subtilis QST713 decreased quickly following application and did not establish a persistent community16, whereas directing QST713 applications to the rhizosphere led to a persistent population that increased over time.10 Considering QST713 was initially isolated from peach tree soil, it makes sense that it can establish a persistent population in the rhizosphere.17 While populations of a biological control organism can be maintained through a growing season with frequent applications, additional research for sustaining applied biological control organism populations is needed. Efforts may be more fruitful if the native environment of the biological control organism is given more consideration when trying to implement a biological in disease management.
Populations are not the only thing to consider with biological control organisms. Reducing disease severity is the goal. Recently published research continues to show mixed results. Weekly applications of QST713 reduced copper spot severity on creeping bentgrass fairway, but dollar spot and brown patch severity were unaffected.16 Pythium root rot was only reduced by a biological control organism under low disease pressure.10 Interestingly, biological control organisms did reduce microdochium patch severity in Oregon, where microdochium patch is very aggressive.18 Under low to moderate levels of dollar spot pressure, biological control organisms and a mineral oil-based product reduced disease severity to similar levels as the fungicide boscalid (e.g., Emerald), but not on all rating dates throughout the trial periods.19 One fact held true across all these studies; disease severity was reduced compared to the non-treated control only, whereas synthetic fungicides reduced disease severity more than the biological control organisms.
Adoption Considerations
Applications of biological control organisms need to be started preventatively and made at the highest label rate on the shortest interval allowed by the label. This will help maintain higher populations of the applied biological control organism by the time favorable conditions for disease development begin. While higher populations will not guarantee increased disease reduction, maintaining higher populations of a biological control organism will increase the chance of success and possibly suppress less competitive pathogens (such as the copper spot pathogen) in areas where fungicides are not used.
Biological control cannot succeed under periods of high disease pressure. Perhaps this is due to the pathogen overwhelming the biological control organism or due to the biological control organism not being at sufficient populations throughout the disease epidemic. This is where additional work is needed by researchers. If we hope to reduce reliance on synthetic fungicides and increase the adoption of biological control organisms, there needs to be an increased understanding of the population dynamics and interactions between pathogens and biological control organisms.
Finally, there is a need to manage expectations surrounding biological control organisms and biological management of turfgrass diseases. Perhaps it was overzealous claims made early in the life of biological control organisms, generous marketing claims, or a failure on the part of the research community to publish and discuss research with “negative” or non-significant results more regularly. Regardless of the underlying cause, it is important to communicate that biological control organisms cannot, and should not, be thought of or used as drop-in replacements for pesticides in a pest management program. A complete switch to biological turfgrass disease management is not feasible in many cases. For residential landscape contractors, numerous properties with busy schedules make weekly applications nearly impossible, as most lawn care operations make spray applications once per month or quarterly. Similarly, budgetary and manpower constraints can make weekly applications impossible for smaller area golf courses and/or sports facilities. If budgets allow, the adoption of biological control organism programs may be attainable in targeted areas of turfgrasses like sports fields and/or golf courses that are accustomed to making weekly maintenance applications.
The best disease management programs do not rely on synthetic fungicides alone. The same is true for implementing biological-based programs. Due to the numerous unknowns surrounding microbial interactions in the plant microbiome, biological control organisms will inherently have significantly more variation in their suppression than synthetic fungicides (figure 2). Practicing proper management techniques and implementing an integrated pest management program will be essential to maximize success when utilizing biological control organisms.
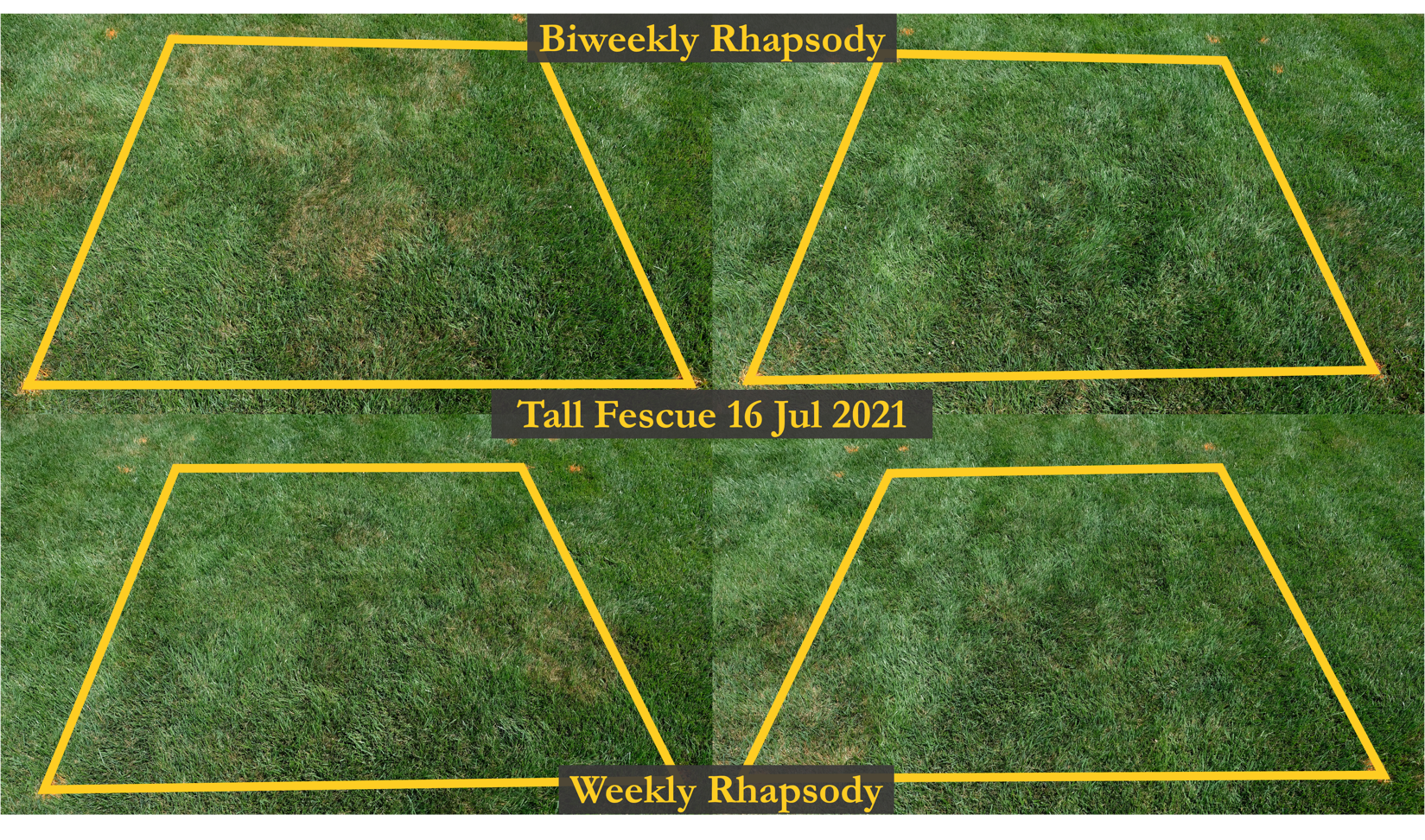
Figure 2. Variation in the efficacy of biological control organisms in reducing brown patch in a tall fescue home lawn field trial at the peak of disease severity. Images are of high disease severity (left), low disease severity (right), biweekly Bacillus subtilis QST713 (Rhapsody) applications (top), and weekly B. subtilis QST713 applications (bottom). Image credit: Joseph Doherty.
References Cited
- Jeffers AH, Chong JC. Biological control strategies in integrated pest management (IPM) programs. Clemson (SC): Clemson Cooperative Extension, Land-Grant Press by Clemson Extension; 2021. LGP 1111. http://lgpress.clemson.edu/publication/biological-control-strategies-in-integrated-pest-management-programs.
- Baker K. Evolving concepts of biological control of plant pathogens. Annual Review of Phytopathology. 1985;25(1):67–85.
- Whipps J. Microbial interactions and biocontrol in the rhizosphere. Journal of Experimental Botany. 2001 Mar;52(suppl_1):381–401.
- Geraldine A, Lopes F, Carvalho D, Barbosa E, Rodrigues A, Brandão R, Ulhoa J, Lobo Jr M. Cell wall-degrading enzymes and parasitism of sclerotia are key factors on field biocontrol of white mold by Trichoderma spp. Biological Control. 2013 Dec;67(3):308–316.
- Arya N, Rana A, Rajwar A, Saghal M, Sharma A. Biocontrol efficacy of siderophore producing indigenous Pseudomonas strains against fusarium wilt in tomato. National Academy of Sciences Letters. 2018 Jun;41(3):133–136.
- Meziane H, van der Sluis I, van Loon L, Höfte M, Bakker P. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Molecular Plant Pathology. 2005 Mar;6(2):177–185.
- Miller M, Bassler B. Quorum sensing in bacteria. Annual Reviews in Microbiology. 2001 Oct;55(1):165–199.
- Hamoen L, Venema G, Kuipers O. Controlling competence in Bacillus subtilis: Shared use of regulators. Microbiology. 2003 Jan;149(Pt 1):9–17.
- Liu X, Bimerew M, Ma Y, Muller H, Ovadis M, Eberl L, Berg G, Chernin L. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiology Letters. 2007 May;270(2):299–305.
- Doherty J , Roberts J. Investigating chemical and biological control applications for pythium root rot prevention and impacts on creeping bentgrass putting green rhizosphere bacterial communities. Plant Disease. 2022 Feb;106(2):641–647.
- Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA. Principles and applications of soil microbiology. 2 ed. Upper Saddle River (NJ): Pearson Prentice Hall; 2005. 640 p.
- Tredway LP, Tomaso-Peterson M, Kerns JP, Clarke BB. Compendium of turfgrass diseases. 4 ed. St. Paul (MN): American Phytopathological Society (APS Press); 2023.
- Latin R. Seasonal activity of turfgrass pathogens in the Midwest. West Lafayette (IN): Purdue Extension, Professional Series; 2013 Mar. BP-125-W.
- Schumann GL, D’Arcy CJ. How do people influence plant disease epidemics? In: Schumann GL, D’Arcy CJ, editors. Essential plant pathology. St. Paul (MN): American Phytopathological Society (APS Press); 2006. p. 227–254.
- Latin R. Biofungicides, phosphonates, and post-patent products. In: Latin R. A practical guide to turfgrass fungicides. St. Paul (MN): American Phytopathological Society (APS Press); 2011. p. 107–121.
- Doherty J, Roberts J. Topdressing biochar compost mixtures and biological control organism applications suppress foliar pathogens in creeping bentgrass fairway turf. Plant Disease. Forthcoming 2023.
- European Food Safety Authority, Anastassiadou M, Arena M, Auteri D, Brancato A, Bura L, Cabrera LC, Chaideftou E, Chiusolo A, Crivellente F, et al. Peer review of the pesticide risk assessment of the active substance Bacillus amyloliquefaciens strain QST 713 (formerly Bacillus subtilis strain QST 713). EFSA Journal. 2021 Jan;19(1):e06381.
- Mattox C, Kowalewski A, McDonald B, Lambrinos J, Pscheidt J. Rolling and biological control products affect microdochium patch severity on a sand-based annual bluegrass putting green. Agronomy Journal. 2018 Nov;110(6):2124–2129.
- Koch P, Hockemeyer K, Buczkowski E. Evaluating biological and oil-based fungicides for dollar spot suppression on turfgrass. Agronomy Journal. 2020 Sep;105:3808–3818.
Additional Resources
Jeffers AH, Chong JH. Biological control strategies in integrated pest management programs. Clemson (SC): Clemson Cooperative Extension, Land-Grant Press by Clemson Extension; 2021. LGP 1111. http://lgpress.clemson.edu/publication/biological-control-strategies-in-integrated-pest-management-programs.

