Cotton growers grapple with regrowth challenges post-defoliation, leading to diminished fiber quality and economic returns. Chemical treatments, while effective, depend on various factors such as growing season conditions, soil moisture, fertility, temperature, defoliant application timing, and harvest scheduling. By exploring molecular breeding approaches, the goal is to develop cotton genotypes that minimize or eliminate regrowth, complementing and reducing chemical treatments. This publication aims to provide cotton producers, researchers, Extension Agents, and specialists with insights into cotton regrowth genetics and physiology. For those who may need more of an introduction to the importance of cotton physiology and genetics, resources such as Ritchie et al.1 and the Genetic Science Learning Center2 will help build a baseline of knowledge for the content of this publication.
Introduction
Cotton (Gossypium spp.) is a primary source of natural fiber and a contributor to the agricultural economy of many countries around the globe. It produces an economic impact of over $600 billion worldwide.3 The United States produces approximately 15% of the world’s cotton and shares 35% of the world’s export market.4 In the United States, cotton is primarily produced by the southern states, also known as the Cotton Belt. Among these states, Texas is the leading producer, accounting for about 40% of United States cotton production; other leading producers are Georgia, Mississippi, and Arkansas. Although South Carolina produces less than 3% of total United States cotton, it is one of the major crops produced in the state. Cotton was cultivated over 270,000 acres, which yielded 505,000 cotton bales in 2022 (an average of 911 lb. per acre) and generated $248 million in revenue.5 The photoperiod-insensitive Upland cotton (Gossypium hirsutum) genotypes were cultivated throughout the United States Cotton Belt. Some acreage in California, Arizona, and Texas is dedicated to the production of Pima cotton (Gossypium barbadense). Upland cotton is cultivated for its high productivity and average fiber quality; however, Pima cotton is produced specifically for high fiber quality.
Growth and Defoliation
Cotton is a perennial plant (a persistent plant that continues to regrow from its base) with an indeterminate growth habit, which means plants continue to produce more growth from the apical meristem (a set of undifferentiated juvenile cells capable of producing new growth at shoot and root tips by repeated cell divisions) as reproductive development begins; the formation of a floral bud does not consume the apical or branch meristem.6,7 Since indeterminate cotton plants continue to grow vegetatively, producers must terminate their cotton crop by spraying harvest aid products that remove mature and juvenile leaves, inhibit regrowth, and expedite boll opening to facilitate once-over mechanical harvesting. In the United States, the most widely used defoliation active ingredients include tribufos (a cell wall disrupter), diuron (a photosynthesis inhibitor), thidiazuron (an auxin inhibitor), and ethephon (a plant growth regulator). The widespread use of these defoliants raises environmental and public health concerns, much like the use of pesticides and herbicides.8,9
Pests and Pathogens
Regrowth after defoliation causes reduced fiber quality from increased lint staining, increased trash, and reduced harvest efficiency (the modules and trailers heat up if the moisture content is more than 12% or if less than 80% of leaves are removed). Regrown tissues can also provide food sources for insect pests and serve as a host for various pathogens.10 For example, the southern root-knot nematode is a damaging parasite of cotton that, in combination with cotton regrowth, causes significant economic losses.11
The losses to the southern root-knot nematode across the United States Cotton Belt alone were estimated to be 2.2% or 414,700 cotton bales in 2016.12 The 2nd stage juvenile (J2) of this nematode penetrates cotton root tips. Once J2 is established in vascular tissue, it does not move and ultimately completes its life cycle inside the roots. Cotton harvest in the southeastern United States typically initiates in September or October. When soil temperatures are still above the threshold level required for their development, southern root-knot nematodes that have already entered roots and established feeding sites continue to reproduce and grow. Hence, the population increases over time. If cotton stubble is left on the ground, it supports regrowth under specific conditions (highlighted in the subsequent section) and serves as a breeding ground for pests and pathogens.
Regrowth
It is often difficult to control regrowth in cotton due to its indeterminate nature, and preharvest and postharvest regrowth can reduce fiber quality and economic returns to cotton producers. Since chemical treatments are not always efficient in limiting regrowth, it may be possible to alter the physiological and genetic aspects that determine regrowth and use this knowledge to reduce the losses associated with preharvest and postharvest regrowth.
What is Regrowth?
As cotton bolls mature and dehisce, they no longer draw nutrients from photosynthetic source tissues (i.e., leaves, stems, and bracts), allowing photosynthates to accumulate in the plant.13 This accumulation of additional nutrients and carbohydrates often induces a second round of vegetative growth, commonly known as “regrowth.” Regrowth is regularly observed in most cotton fields in South Carolina; however, different varieties appear to exhibit different levels of regrowth potential after defoliation. Regrowth is genetically regulated and highly influenced by environmental factors.14 The regrowth can include multiple types, such as juvenile leaves and fruit in the upper canopy and on outer positions of fruiting branches, as well as basal regrowth initiating from the main stem. The economic importance of boll to producers and the effectiveness and cost of chemical control to manage regrowth support the need for genetic control to supplement chemical treatments.15,16
Physiological Aspects of Regrowth
The most important environmental factors that induce/promote regrowth in cotton are high temperature, ample day length (sunlight), and excessive soil moisture and fertility at harvest.
Warmer Growth-Promoting Temperatures
The temperature, light quality, and light quantity are critical factors determining plant growth and development. Each plant species has a range of minimum, maximum, and optimum temperatures for growth.13 Cotton has an optimum daytime temperature of 82°F ± 6°F for various processes and growth stages.17
In the vegetative (non-flowering/fruiting) phase, the plant produces energy that sustains the development of vegetative organs (leaves, stems, bracts, and roots) and later squares, flowers, and bolls during the reproductive phase (figure 1).18 The node and leaf appearance rate (vegetative growth) increases in plants as temperatures rise to optimum growth temperature. According to a recent study, new leaves emerge as a linear function of time at any temperature regime.19,20 As the temperature increases, the time interval between the appearance of the two successive leaves decreases.21–23 Increasing temperature accelerates the leaf growth rate until the optimum temperature is reached, but a further rise in temperature reduces leaf growth.24,25

Figure 1. Cotton growth stages over the course of its development. Image credit: ilyakalinin – stock.adobe.com.

Figure 2. Plot showing the relationship between cotton leaf expansion and temperature. Image credit: Adapted from Reddy et al.26
Temperature also regulates leaf expansion, especially in cell-expansion zones. The surface area of the cotton plant leaves increases as they emerge and develop, resulting in an increase in sunlight interception. As a result of sunlight interception, the leaves produce carbohydrates that are translocated, increasing the growth of roots and leaves. Due to the production of new leaves in the plant canopy, a relatively higher net photosynthetic rate was observed at an average daily mean temperature of 80.6°F relative to the plants grown at 62.6°F or lower temperatures. Additionally, the leaves expand more rapidly at 86°F than at 68°F or 98.6°F (figure 2).26 Therefore, it is expected that in a warmer and high CO2 environment, the plant’s photosynthetic rates would be higher, producing more vegetative growth.
Cotton is primarily grown in the Cotton Belt of the southern United States (figure 3A). During the fall months, particularly in September and October, temperatures in cotton-producing states average between 69.8°F and 91.4°F (figure 3B). Warm fall temperatures and ample light duration and intensity in the southern United States promote and support regrowth. Consequently, plants that have not expressed their full genetic potential due to environmental or other factors during the growing cycle exhibit variable regrowth levels, depending on their genetics. In some states, postharvest cotton stalks remain in the field for almost 140 days. The gradual increase in temperature from March to April can lead to increased leaf production on the cotton stalks, and these continued growth-promoting conditions can further complicate crop management.

Figure 3. The map in 3A identifies cotton production areas in the United States,27 and the graph in 3B shows maximum temperatures during fall months (September to November) in the United States Cotton Belt.28 Image credits: USDA National Agricultural Statistics Service (A), NOAA National Centers for Environmental Information (B).
Light Penetration
The photosynthetic rate in leaves does not remain constant and varies as leaves grow. Further, the photosynthetic activity in plants is associated with many factors, including the optimal use of light, which is influenced by light penetration through the canopy.29 Plant leaf shape, leaf size, and canopy architecture also affect photosynthetic efficiency.30 In defoliated plants, photosynthesis was enhanced mainly due to better light penetration in the canopy (i.e., light reaching axillary buds at the base of each branch), leading to 10% to 18% more average photosynthesis per unit leaf area than in untreated plants.31 Hence, the defoliant application opens up the canopy, allowing light to reach the base of each branch, leading to renewed vegetative growth.
Leaf orientation substantially affects light penetration into plant canopies. Change of leaf orientation in response to the change in the sun’s direction occurs in many plants, including cotton.32 This solar tracking movement (heliotropism) is generally classified into two types: diaheliotropism (leaves oriented perpendicular to the sun’s rays) and paraheliotropism (leaves oriented parallel to the sun’s rays to minimize excess light absorption). In Upland cotton, the perpendicular leaf orientation results in higher leaf irradiance and, thereby, higher photosynthesis.33,34
Soil Moisture and Fertility
Irrigation is stopped before harvest, which results in low soil moisture that forces cotton stalks to compete for moisture. Root growth and distribution in the soil profile are influenced by abiotic and biotic factors such as soil water availability and defoliation.35 Immediately after defoliation, the effects of reduced canopy photosynthesis rapidly spread throughout the plant. Root respiration begins to decline within hours of defoliation and decreases substantially within twenty-four hours.24,36,37 Since regrowth starts with limited plant tissue, there is little demand for water; therefore, even marginal soil moisture can support regrowth.
As root respiration diminishes following defoliation, so does nutrient uptake. A fertile soil consists of different levels of plant growth-limiting nutrients like nitrogen, phosphorus, potassium, and organic matter.39,40 Although low soil fertility can negatively impact cotton production,41-43 regrowth has limited leaf or surface area, so any remaining soil nutrients can still provide sufficient nutrients to cotton stalks, inducing and sustaining regrowth.
Source-Sink Relationship
In cotton, the rate of vegetative growth (i.e., adding more mainstem nodes with subtending leaves, flowers, or fruiting branches) slows as boll development begins.44 Later in the developmental process, reproductive growth overtakes vegetative growth. This specific developmental time point in cotton is known as “cutout.” In a more practical sense, cutout is defined as a developmental timepoint when the plant develops a white flower at the first position (i.e., the fruiting site closest to the mainstem on a fruiting branch) on the sixth node from the top (figure 4). From this point in development, the cotton crop becomes more source-limited (i.e., limited by carbohydrate availability), in contrast to when there was less vegetative growth and a surplus of resources. Generally, in cotton, by the time the first fruiting branches initiate flowering, there are eight or more internodes on the mainstem, and from that developmental stage, node development continues until cutout. Thereafter, few, if any, subsequent fruit contribute significantly to harvested yield.
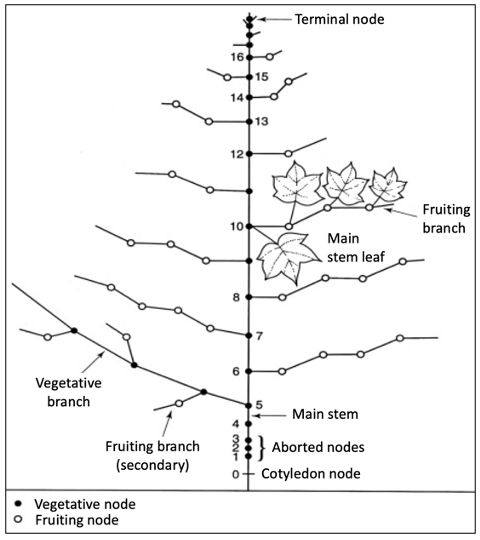
Figure 4. Diagrammatic representation of a typical cotton plant. Image credit: Adapted from John et al.45 (Originally adapted from Oosterhuis DM. Growth and development of the cotton plant. 1990.)
An optimally yielding cotton variety often produces twenty to twenty-five mainstem nodes, and twelve to eighteen develop into fruiting branches. After cutout, young fruit generally shed due to competition for resources with maturing fruit (figure 5). In cotton, more than 90% of fruits from the first fruiting branches are retained compared to less than 20% retained from the upper branches. Climatic conditions, including heat stress, drought, and erratic rain patterns, can cause cotton plants to shed fruit and often trigger a reversal to vegetative growth (regrowth). This phenomenon occurs in years that do not support large boll loads (higher yields), where extensive regrowth is observed. In contrast, no regrowth was observed in years that supported high yields and substantial boll set.

Figure 5. Sensitivity of different boll developmental stages to shedding. Image credit: Adapted from National Cotton Council of America.43
Cotton is particularly sensitive to high temperatures during the reproductive phase. The total number of fruiting branches in cotton increases by 50% with an increase in temperature from 86°F to 104°F; however, boll retention drops sharply at temperatures above 95°F to zero at 104°F due to enhanced abortion of squares and young bolls.46 Cotton plants exposed to a 96.8°F daytime temperature and 82.4°F nighttime temperature retained approximately 70% fewer bolls than plants grown at an 86°F daytime temperature and 71.6°F nighttime temperature.46 The effect of heat stress was consistent across Pima and Upland cotton. However, Pima cotton appears to be relatively more tolerant to higher temperatures than Upland cotton.47 To summarize, boll retention and maturation are negatively associated with heat stress at the reproductive stage.
Genetic Aspect of Regrowth
Meristems, both primary and secondary, are small groups of undifferentiated cells that give rise to the plant body. The primary meristems are established during embryo development and form primary tissues from which all plant organs originate. Secondary meristems, such as axillary meristem (bud meristem) and the cambium (lateral meristem), develop within primary tissues. Meristems can be determinate (active until the production of a specific plant part of predictable form and size [e.g., a flower]) or indeterminate (stay active throughout the life of the plant). In annual plants (die after a growing season), at the time of floral induction, vegetative shoot meristems undergo the transition to inflorescence (a flower cluster) meristems, which stay indeterminate (i.e., continue to add leaves and flowers) for some time and eventually give rise to the determinate floral meristems.48 Finally, all meristems get consumed, and the plant dies in the same growing season. In contrast, perennial plants have evolved more elaborate life strategies to survive for many years by forming perennial structures such as buds, bulbs, or tubers, which contain at least one indeterminate meristem for outgrowth in the next season.19,49
Annual plants have evolved many times from perennial ancestors, and the reverse has also occurred, albeit to a limited extent. These life history traits and histories have resulted in the evolution of closely related species with different growth habits: annual and perennial. The differential behavior of meristems, whereby some stay vegetative while others transition to flowering, is an important aspect that distinguishes perennials from annual plants.
Genes that control the determinacy of meristems have been found in various plants.19,49–52 The mutant types of these genes can alter the growth habit of a plant from indeterminate to more determinate type by producing more annual characteristics such as early flowering, erect plant architecture, reduced preharvest and postharvest vegetative growth (figure 6).53–56
In cotton, a network of similar genes may be utilized to alter the growth habit of a cotton plant from indeterminate to a more determinate type with more annual type characteristics, such as reduced regrowth. With these changes in the growth habit, the cotton plant would be expected to efficiently remobilize reserves to the terminal or sink tissues (seeds and seed fibers), maintain a compact stature, and complete its life cycle in a single growing season. Cultivated Upland cotton is a perennial with an indeterminate growth habit that reserves storage compounds in its primary body to support growth in the next growing season and uses reserves to promote secondary growth (i.e., thickening of stems or roots) to withstand future environmental challenges. These reserves, if transported to the sink tissues, may translate into higher yield. Cotton bred precisely for reduced regrowth may focus carbohydrate resources toward yield and die, minimizing the need for defoliant application.

Figure 6. Indeterminate (wild type) shown in A. Determinate (tfl1/sp mutant) shown in B. Mutations in the FLOWERING LOCUS T (FT) antagonist TERMINAL FLOWER 1 (TFL1) gene suggested that cotton plants carrying high expression alleles of the FT gene might exhibit a somewhat similar phenotype (i.e., with clustered flowering). Image credit: Adapted from Eshed and Lippman.56
Scientists at Clemson University’s Pee Dee Research and Education Center identified Upland cotton genotypes with the high-expression variants of the five floral induction and meristem identity genes from the United States cotton mini-core collection (representatives of the Upland cotton genotypes bred in the last 100 years). They stacked them by genetic crossing in a single genotype to develop cotton genotypes with variable degrees of determinate growth habit with improved yield and reduced regrowth.23,49,57 These genotypes were selected for reduced regrowth after defoliation and advanced to F9:10 generation (filial generation obtained after genetic crossing using a single seed descent method), and advanced lines were evaluated under replicated field trials in the last two years.23 These genotypes are being re-evaluated for the third year in 2024 before being considered for release as germplasm. This effort differs from earlier attempts in cotton, where the FT gene (florigen) was transiently expressed using a viral construct, or the FT antagonist TFL1 (anti-florigen) was silenced. These activities resulted in very severe phenotypes, where either (1) fruiting branches initiated from earlier nodes of the main stem and terminated prematurely with clusters of flowers instead of initiating the next flowering branch or (2) produced no vegetative (third node from the cotyledonary node) or fruiting branches, as all vegetative and fruiting branch meristem of the main axis and axillary meristems (buds in leaf axils) terminated into flowers.58
Summary
The current approach of Clemson University researchers is to act on cotton architecture to maximize yield via channeling resources (assimilates or carbohydrates) towards developing bolls by tweaking cutout timing. These genotypes are expected to reduce the amount of plant growth regulators used to alter the architecture or terminate the crop, maximize fiber yield, avoid regrowth, and subsequently improve economic returns.
Acknowledgments
The authors would like to acknowledge the support for this work from the South Carolina Cotton Board and Cotton Incorporated.
References Cited
- Ritchie GL, Bednarz CW, Jost PH, Brown SM. Cotton growth and development. Athens (GA): The University of Georgia, College of Agricultural and Environmental Sciences; 2004. [accessed 2024 Jun 30]. https://esploro.libs.uga.edu/esploro/outputs/report/Cottongrowth-and-development/9949316286902959#file-0.
- Why Study Cotton Genes? Salt Lake City (UT): Genetic Science Learning Center; 2010 [accessed 2024 Aug 10]. https://learn.genetics.utah.edu/content/cotton/genes/.
- Ahmad S, Hasanuzzaman M. Cotton production and uses: agronomy crop protection, and postharvest technologies. New York (NY): Springer Nature, Springer Singapore; 2020.
- Ridley W, Devadoss S. Competition and trade policy in the world cotton market: implications for US cotton exports. American Journal of Agricultural Economics. 2023 Jan;105(5):1365–1387. doi:10.1111/ajae.12370.
- 2023 State Agriculture Overview, South Carolina. Washington (DC): United States Department of Agriculture (USDA), National Agricultural Statistics Services (NASS); 2023. [accessed 2024 Jun 30]. https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=SOUTH%20CAROLINA.
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275(5296):80–83. doi:10.1126/science.275.5296.80.
- Tian Z, Wang X, Lee R, Li Y, Specht JE, Nelson RL, Ma J. Artificial selection for determinate growth habit in soybean. Proceedings of the National Academy of Sciences (PNAS). 2010 Apr;107(19):8563–8568. doi:10.1073/pnas.1000088107.
- Scarborough ME, Ames RG, Lipsett MJ, Jackson RJ. Acute health effects of community exposure to cotton defoliants. Archives of Environmental and Occupational Health. 1989 Nov–Dec;44(6):355–360. doi:10.1080/00039896.1989.9935906.
- Ma M, Ayaz P, Jin W, Hussain M, Zhou W. Analysis of the dissipation behavior of defoliants in cotton fiber during field and scouring process using liquid and gas chromatography. International Journal of Analytical Chemistry. 2019;2019(1):2879074. doi:10.1155/2019/2879074.
- Ribeiro EB, Castellani MA, Silva CA, Melo TL, Silva GD, Vale WS, Santos AS. Métodos de destruição de restos de cultura do algodoeiro e sobrevivência do bicudo. Pesqui Agropecu Bras. 2015;50(11):993–998. doi:10.1590/s0100-204×2015001100001.
- Carter E. Integrated pest management: importance of managing cotton regrowth. Gainesville (FL): University of Florida, IFAS Extension; 2019. https://nwdistrict.ifas.ufl.edu/phag/2019/11/15/integrated-pest-management-importance-of-managing-cotton-regrowth/#:~:text=Cotton%20regrowth%20after%20stalks%20were,on%20during%20the%20winter%20months.
- Dyer DR, Lawrence K, Schrimsher D. Yield loss to cotton cultivars due to reniform and root-knot nematode and the added benefit of velum total. In: Boyd S, Huffman M, Krogman L, Sarkissian A, editors, 2018 Proceedings. Beltwide Cotton Conferences, 2018 Jan 3–5, San Antonio (TX): National Cotton Council; c2013. p. 511–514.
- Hatfield JL, Prueger JH. Temperature extremes: effect on plant growth and development. Weather and Climate Extremes. 2015 Dec;10:4–10. doi:10.1016/j.wace.2015.08.001.
- Naveed S. Breeding cotton for determinate growth habit: increasing cotton fiber yield by remobilization of end-of-season perennial reserves [dissertation]. Clemson (SC): Clemson University, Department of Plant and Environmental Science; 2023. [accessed 2024 Feb 25]. https://tigerprints.clemson.edu/all_dissertations/3431.
- Byrd S, Wilson B, Catlin C, Althoff A. 2021 Oklahoma cotton harvest aid guide. Stillwater (OK): Oklahoma State University; 2021 Sep 16 [accessed 2024 Feb 25]. https://extension.okstate.edu/fact-sheets/2021-oklahoma-cotton-harvest-aid-guide.html.
- Raper T. Cotton specialist P. Key cotton growth and development stages. Knoxville (TN): University of Tennessee, Institute of Agriculture; [accessed 2024 Feb 25]. https://www.planthealthexchange.org/cotton/Documents/GROW-COT-04-21-295.pdf.
- Burke JJ, Wanjura DF. Plant responses to temperature extremes. In: Stewart JM, Oosterhuis DM, Heitholt JJ, Mauney JR, editors. Physiology of cotton. Dordrecht (NL): Springer; 2010. p. 123–128. doi:10.1007/978-90-481-3195-2_12.
- Naveed S, Toyinbo J, Ingole H, Valavanur SP, Jones M, Campbell BT, Rustgi S. Development of high-yielding upland cotton genotypes with reduced regrowth after defoliation using a combination of molecular and conventional approaches. Genes. 2023;14(11):2081. doi:10.3390/genes14112081.
- Tamaki M, Kondo S, Itani T, Goto Y. Temperature responses of leaf emergence and leaf growth in barley. The Journal of Agricultural Science. 2002 Feb;138(1):17–20. doi:10.1017/s0021859601001745.
- Bartholomew PW, Williams RD. Cool-season grass development response to accumulated temperature under a range of temperature regimes. Crop Science. 2005;45(2):529–534. doi:10.2135/cropsci2005.0529.
- Kirby EJM. Factors affecting rate of leaf emergence in barley and wheat. Crop Science. 1995 Jan;35(1):11–19. doi:10.2135/cropsci1995.0011183X003500010003x.
- Bos HJ, Neuteboom JH. Growth of individual leaves of spring wheat (Triticum aestivum L.) as influenced by temperature and light intensity. Annals of Botany. 1998 Jan;81(2):141–149. doi:10.1006/anbo.1997.0532.
- McMaster GS, Wilhelm WW, Palic DB, Porter JR, Jamieson PD. Spring wheat leaf appearance and temperature: extending the paradigm? Annals of Botany. 2003 May;91(6):697–705. doi:10.1093/aob/mcg074.
- Clement CR, Hopper MJ, Jones LHP, Leafe EL. The uptake of nitrate by Lolium perenne from flowing nutrient solution. II. Effects of light, defoliation, and relationship to CO2 flux. Journal of Experimental Botany. 1978 Oct;29(3):1173–1183. doi:10.1093/jxb/29.5.1173.
- Cao W, Moss DN. Temperature effect on leaf emergence and phyllochron in wheat and barley. Crop Sci. 1989 Jul–Aug;29(4):1018–1021. doi:10.2135/cropsci1989.0011183x002900040038x.
- Reddy KR, Hodges HF, McCarty WH, McKinion JM. Weather and cotton growth: present and future. Mississippi State (MS): Mississippi State University, Division of Agriculture, Forestry, and Veterinary Medicine; 1996 Oct. Bulletin 1061. https://www.mafes.msstate.edu/publications/bulletins/b1061.pdf.
- United States: Cotton Production. Washington (DC): U.S. Department of Agriculture (USDA), National Agricultural Statistics Service (NASS); 2020 [accessed 2022 Nov 19]. https://ipad.fas.usda.gov/rssiws/al/crop_production_maps/US/USA_Cotton.png.
- Climate Monitoring: U.S. Maps. Silver Spring (MD): National Oceanic and Atmospheric Administration (NOAA), National Centers for Environmental Information. https://www.ncei.noaa.gov/access/monitoring/us-maps/maps?maps=statewide-tmax-rank–1–202209.
- Song Q, Zhang G, Zhu XG. Optimal crop canopy architecture to maximize canopy photosynthetic CO2 uptake under elevated CO2–a theoretical study using a mechanistic model of canopy photosynthesis. Functional Plant Biology. 2013 Mar;40(2):108. doi:10.1071/fp12056.
- Alarcon VJ, Sassenrath GF. Optimizing canopy photosynthetic rate through par modeling in cotton (Gossypium spp.) crops. Computers and Electronics in Agriculture. 2015 Nov;119:142–152. doi:10.1016/j.compag.2015.10.010.
- Anten NPR, Ackerly DD. Canopy-level photosynthetic compensation after defoliation in a tropical understorey palm. Functional Ecology. 2001 Apr;15(2):252–262. doi:10.1046/j.1365-2435.2001.00517.x.
- Ehleringer J, Forseth I. Diurnal leaf movements and productivity in canopies. In: Russell G, Marshall B, Jarvis PG, editors. Plant canopies: their growth, form and function. Cambridge (UK): Cambridge University Press; 1989. p. 129–142. doi:10.1017/cbo9780511752308.008.
- Ehleringer JR, Hammond SD. Solar tracking and photosynthesis in cotton leaves. Agricultural and Forest Meteorology. 1987 Jan;39(1):25–35. doi:10.1016/0168-1923(87)90013-x.
- Sassenrathcole G. Dependence of canopy light distribution on leaf and canopy structure for two cotton (Gossypium) species. Agricultural and Forest Meteorology. 1995 Nov;77(1–2):55–72. doi:10.1016/0168-1923(95)02238-s.
- Simoes M, Baruch Z. Responses to simulated herbivory and water stress in two tropical C4 grasses. Oecologia. 1991 Oct;88(2):173–180. doi:10.1007/bf00320808.
- Davidson JL, Milthorpe FL. Leaf growth in Dactylis glomerate following defoliation. Annals of Botany. 1966 Apr;30(2):173–184. doi:10.1093/oxfordjournals.aob.a084065.
- Thorgeirsson H. The modeling and measurement of respiratory carbon use and net carbon gain of two Agropyron bunchgrasses [dissertation]. Logan (UT): Utah State University; 1988. doi:10.26076/8daf-12bd.xc
- Xiong H, Tang Y, Ren D, Li X, Cheng K, Yao W. Studies on relationships between different soil types and climate conditions and grain yield of rice. Southwest China Journal of Agricultural Sciences. 2003 Jan;17:305–309. doi:10.3969/j.issn.10014829.2004.04.009. (In Chinese, with English abstract).
- Zingore S, Murwira HK, Delve RJ, Giller KE. Influence of nutrient management strategies on variability of soil fertility, crop yields and nutrient balances on smallholder farms in Zimbabwe. Agriculture, Ecosystems & Environment. 2007 Feb;119 (1–2):112–126. 2007. doi:10.1016/j.agee.2006.06.019.
- Das A, Prasad M, Gautam R, Shivay Y. Productivity of cotton (Gossypium hirsutum) as influenced by organic and inorganic sources of nitrogen. Indian Journal of Agricultural Sciences. 2006;76(6):354–357.
- Singh RJ, Ahlawat I. Effects of transgenic cotton-based cropping systems and their fertility levels on succeeding wheat crop. Communications in Soil Science and Plant Analysis. 2014 Sep;45(18):2385–2396. doi:10.1080/00103624.2014.912291.
- Kintché K, Guibert H, Sogbedji J, Levêque J, Bonfoh B, Tittonell, P. Long-term mineral fertiliser use and maize residue incorporation do not compensate for carbon and nutrient losses from a Ferralsol under continuous maize–cotton cropping. Field Crops Research. 2015 Dec;184:192–200. doi:10.1016/j.fcr.2015. 04.019.
- National Cotton Council of America. Cotton physiology today. Cordova (TN): National Cotton Council of America. Newsletter of the Cotton Physiology Education Program; 1996 Dec. Vol. 7, No. 7.
- Integrated Crop Management Overview. Cordova (TN): National Cotton Council of America; (n.d.) [accessed 2024 Feb 25]. https://www.cotton.org/tech/ace/upload/Integrated-Crop-Management.pdf.
- John S, Mike B, Jim H. Chapter 22 – Cotton. In: Sadras VO, Calderini DF, editors. Crop physiology case histories for major crops. Cambridge (MA): Academic Press; 2021. p. 714–746. ISBN 9780128191941. doi:10.1016/B978-0-12-819194-1.00022-0.
- Zhao D, Reddy KR, Kakani VG, Koti S, Gao W. Physiological causes of cotton fruit abscission under conditions of high temperature and enhanced ultraviolet-B radiation. Physiologia Plantarum. 2005 May;124(2):189–199. doi:10.1111/j.1399-3054.2005.00491.x.
- Hodges HF, Reddy KR, JM McKinnon, Reddy VR. Temperature effects on cotton. Mississippi (MS): Mississippi State University, Agricultural & Forestry Experiment Station; 1993 Feb. Bulletin 990. https://www.mafes.msstate.edu/publications/bulletins/b0990.pdf.
- McGarry RC, Prewitt SF, Culpepper S, Eshed Y, Lifschitz E, Ayre BG. Monopodial and sympodial branching architecture in cotton is differentially regulated by the Gossypium hirsutum SINGLE FLOWER TRUSS and SELF-PRUNING orthologs. New Phytologist. 2016 Jun;212(1): 244–258. doi:10.1111/nph.14037.
- Naveed S, Gandhi N, Billings G, Jones Z, Campbell BT, Jones M, Rustgi S. Alterations in growth habit to channel end-of-season perennial reserves towards increased yield and reduced regrowth after defoliation in Upland cotton (Gossypium hirsutum L.). International Journal of Molecular Sciences. 2023;24(18):14174. doi:10.3390/ijms241814174.
- Xiong S, Guo D, Zhao W, Quan L, Lu W, Xue Y, Liu B, Zhai H. Regulation of soybean stem growth habit: A ten-year progress report. Crop Journal. 2023;11(6):1642–1648. doi:10.1016/j.cj.2023.08.002.
- Chen D, Yan W, Fu LY, Kaufmann K. Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nature Communications. 2018 Oct;9:4534. doi:10.1038/s41467-018-06772-3.
- Lilac P, Lea CG, Dana H, Tamar G, John, Martin G, Daniel Z, Eliezer L. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development. 1998 Jun;125(11):1979–1989. doi:10.1242/dev.125.11.1979.
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genetics. 2008 Nov; 40(12):1489–1492. doi:10.1038/ng.253.
- Adrian J, Torti S, Turck F. 2009. From decision to commitment: the molecular memory of flowering. Molecular Plant. 2009 Jul;2(4):628–642. doi:10.1093/mp/ssp031.
- Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA. Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis. The Plant Cell. 2013 Mar;25(3):820–833. doi:10.1105/tpc.113.109355.
- Eshed Y, Lippman ZB. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science. 2019 Sept;366(6466):eaax0025. doi:10.1126/science.aax0025.
- Naveed S, Rustgi S. 2023. Functional characterization of candidate genes, Gohir.D05G103700 and Gohir.D12G153600, identified through expression QTL analysis using virus-induced gene silencing in upland cotton (Gossypium hirsutum L.). Agriculture. 2023 May;13(5):1007. doi:10.3390/agriculture13051007.
- McGarry RC, Ayre BG. Cotton architecture: Examining the roles of SINGLE FLOWER TRUSS and SELF-PRUNING in regulating growth habits of a woody perennial crop. Current Opinion in Plant Biology. 2021 Feb;59:101968. doi:10.1016/j.pbi.2020.10.001.

