Granville wilt (bacterial wilt) is the most destructive, yield-limiting soil-borne disease of flue-cured tobacco in South Carolina.1 The first confirmed report of this disease in the United States was in Granville County, North Carolina, in the 1880s.2 In South Carolina, the disease is concentrated in the Pee Dee Region’s eastern-most counties but is present and increasing in severity in other important tobacco-producing counties.1 When environmental conditions are conducive to disease development, field losses can range between 25% to 100% (figure 1).3
Pathogen
Granville wilt is caused by a bacterial pathogen Ralstonia solanacearum and affects as many as 197 species in 33 families of plants worldwide.4 The pathogen occurs most prominently in the warm temperate, semitropical, and tropical zones of the world.2 R. solanacearum enters and infects the tobacco plant through wounded roots and can also be mechanically transmitted by farm machinery from an infected plant during topping and harvest operations.5,6
Disease Cycle and Epidemiology
Infection typically occurs via root penetration from wounding caused by cultivation, nematodes, insects, the emergence of secondary roots, etc. When the bacteria enter the xylem vessels, it then spreads within the plant, dissolving cell walls and creating cavities in the pith that ooze with bacteria and cell debris.5 Initially, the clogging of the plant’s vascular system causes a restriction in the flow of water and nutrients. Symptom expression is affected by plant stressors like temperature and water availability.
Symptoms on Tobacco
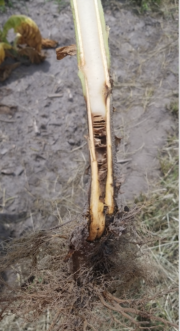
Unilateral wilting and yellowing are the most common symptoms of Granville wilt (figure 2). During the heat of the day, one or two leaves on the bottom portion of one side of the plant (i.e., unilateral) will begin to wilt or “droop,” but as temperatures cool in the evening, this wilting may be followed by some degree of recovery. As the bacteria continue to colonize, the leaves will remain wilted and transition from a lighter green to pale yellow and then brown (figure 3). These wilted leaves are more susceptible to sun scald (necrotic areas between the leaf veins) compared to healthy leaves. Internal symptoms include a light-tan to yellow-brown coloring of the vascular system early on and will darken as more tissue is infected. Ultimately, the pith will fully decay, leaving a hollow plant stem near the base of the plant (figure 4).4 An additional and common internal characteristic is the dark brown streaking and darkening between the outer cortex and epidermis of the plant stem (figure 5).7 After plant death, the bacterium is released into the environment to survive for many years on vegetation, soil, and/or water. In advanced stages of decay, a simple bacterial streaming test (jar test) can be conducted in the field to quickly confirm bacterial wilt infection and distinguish it from other vascular wilts caused by fungal pathogens. After placing an infected section of the stem in a jar of water, milky white strands containing bacteria will stream from the xylem tissue of Granville wilt infected samples (figure 6).8
Similar Diseases
Fusarium wilt (Fusarium oxysporum f. sp. nicotianae) is a fungal disease with similar above-ground symptoms to that of Granville wilt. However, fusarium wilt typically has uniform, brown discoloration just beneath the surface of the bark, with the center (pith) remaining unaffected, compared to the streaking or “pencil markings” and decaying pith of Granville wilt (figure 7).Black shank (Phytophthora nicotianae) causes yellowing and wilting of the entire plant and can look similar to advanced stages of Granville wilt infection (figure 8 and 9). However, black shank can also cause “disking” and/or discoloration of the pith (figure 10), a black lesion on the outside of the stalk at the crown, and large lesions on the leaves can occur if spores are splashed up by rain.
Management Recommendations
Granville wilt control cannot be achieved through any one management practice. An integrated pest management (IPM) plan must be implemented if any level of suppression is to be achieved.1,3
Resistant Varieties
Commercial varieties are available with varying levels of resistance to Granville wilt. Selecting varieties with high levels of resistance is the first step in reducing the impact of Granville wilt. The level of disease infestation in a field and the degree of resistance within a variety much be considered. Resistant varieties are not immune and must be included in an integrated disease management program.
Cultural Practices
Crop rotation is critical in order to reduce disease inoculum in the soil. A minimum of three years with non-host crops is best for managing Granville wilt. Suggested rotational crops include soybean, small grains, and other grass crops. Early stalk and root destruction are recommended to promote the decay of plant tissue. If left unattended, plant tissue and debris can serve as a host for disease pathogens.
Chemical Control
There are very limited options for chemical control. Soil fumigants containing chloropicrin (ex. Chlor-O-Pic 100, Pic plus, Telone C-17) are the most common and effective at reducing Granville wilt inoculum. It should be noted that 1,3-dichloropropene (ex. Telone) is used for nematode control and has little to no impact on Granville wilt.
General Management
Avoid wounding plant root system through cultivation, whether too deep or close. Managing insect and nematode populations can also help with root health. Root wounds can create entry points for disease pathogens. Good weed management is important as species such as common ragweed (Ambrosia artemisiifolia) can serve as a host for Granville wilt. Sanitation of equipment and workers is critical. It is recommended to have a sanitation plan for equipment (toppers, harvest equipment, etc.) in between moving from field to field, especially those fields that are known disease areas. Proper alignment of spray and harvest equipment is important to avoid stem abrasions, potentially creating wounds for disease entry.
References Cited
- Peterson PD. South Carolina tobacco grower’s guide. Clemson (SC): Clemson Cooperative Extension; 2017. p. 43–55.
- Kelman A. The bacterial wilt caused by Pseudomonas solanacearum. Technical Bulletin of North Carolina Agricultural Experiment Station. 1953(99).
- Rodriguez RG, Thiessen L. Granville wilt of tobacco. Raleigh (NC): NC State Extension; 2019 (updated 2020 March). https://content.ces.ncsu.edu/granville-wilt-of-tobacco.
- Lucas GB. Diseases of tobacco. Diseases of tobacco. New York (NY): Scarecrow Press; 1958.
- Agrios GN. Identification of a previously unknown disease. In: Plant pathology. 4th ed. San Diego (CA): Academic Press; 1997. p. 39–40.
- Fortnum BA, Gooden D. Transmission and survival of ralstoniasolanacearum on tobacco machinery. Beiträge zur Tabakforschung International/Contributions to Tobacco Research. 2008 Dec;23(3):137-143.
- Todd FA. Flue-cured tobacco: producing a healthy crop. 1st ed. Wendell (NC): Tobacco Consultants; 1981.
- Olson HA. Ralstonia solanacearum. Raleigh (NC): NC State University, Department of Plant Pathology, Pathogen Profiles; Spring 2005. https://projects.ncsu.edu/cals/course/pp728/Ralstonia/Ralstonia_solanacearum.html.