This publication aims to familiarize readers with peanut allergy, the allergic reaction that occurs upon exposure to peanut proteins in susceptible individuals, current therapies, and an affordable remedy under development at Clemson University, specifically the hypoallergenic peanut lines. Additionally, the content updates readers on the current state of research in this landscape elsewhere and discusses potential future directions for managing peanut allergy. Multiple audiences, including peanut-allergic individuals and peanut producers, and those associated with nutrition, food safety, and healthcare issues (e.g., Extension Agents, researchers, dieticians, and relevant policymakers) will benefit from the in-depth information presented below, whether for the purpose of expanding their knowledge or developing a baseline understanding. Terms marked with an asterisk in the text are defined in a Glossary of Terms section at the end of this publication.
Introduction
Peanut (Arachis hypogaea L.), also known as groundnut, is an economically important legume primarily cultivated for seeds in the Southern and Southeastern United States. However, the plant origin is traced back to South America, specifically to a region encompassing Brazil, Bolivia, Argentina, Paraguay, and Uruguay.1 The tetraploid peanut (with two sets of chromosomes from each parent) evolved from a hybridization event between two diploid species (plants with one set of chromosomes from each parent), Arachis duranensis (AA genome donor) and Arachis ipaensis (BB genome donor) in southern Bolivia to northern Argentina region about 4,000 years ago. The tetraploid peanut was cultivated due to its relatively large seeds and other favorable attributes. Around the 16th century, the cultivated peanut spread from its centers of origin in South America to Africa, India, China, and Indonesia. It is believed that the first introduction of the crop occurred in the United States in the 17th century when slave ships arrived from Africa.2 Later, in the 19th century, peanut seeds became a major source of energy for people experiencing poverty.1 It is the fourth most crucial oilseed crop (after soybean, rapeseed, and sunflower) globally and is a vital source of protein for millions in semi-arid and tropical regions. Currently, the crop is produced in the tropical and warm temperature regions of over eighty countries, with the United States leading in the average yield per *hectare2,3 (figure 1).
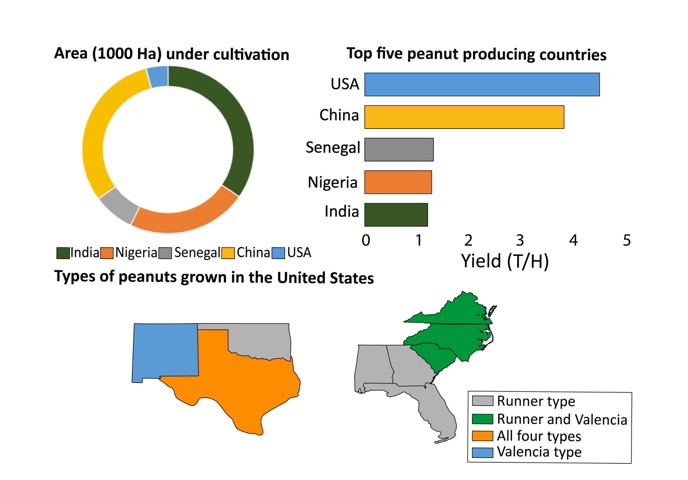
Figure 1. Average peanut production from 2017-2022 in the top five peanut-producing countries. Top left: Area under peanut cultivation (1000 Ha) in India, Nigeria, Senegal, China, and the United States. Top right: Peanut production in the top five peanut-producing countries. The United States is one of the lead peanut producers; however, while the area under peanut production is the least, the per hectare yield (4.46 T/H, 2022) is the highest among the top five peanut-producing nations. Bottom: Map showing the geographical locations where different peanut types are produced in the United States. Image credit: Tariq Alam, Clemson University.
Spanish, Virginia, Valencia, and Runner are four peanut types grown in the United States. The Southeast peanut growing regions primarily produce Runner type, the Virginia-Carolina region grows Virginia and Runner types, and the Southwest region produces Spanish, Valencia, and Runner types (figure 1). Spanish and Virginia market types take 90 to 120 days to produce mature seeds, whereas Runner market type requires 130 to 150 days for seed production after planting.2
Most of the peanuts produced in the United States are used for human consumption. Peanut seed contains 25% to 32% protein and 42% to 52% oil and is also a great source of minerals.2 The bold seeds of the Virginia type are generally sold as in-shell roasted, while the Runner and Spanish types are utilized for peanut butter production, salted cocktails, and candies, and the Valencia type is primarily marketed as medium-sized in-shell nuts.2
Peanut Allergy
Peanut allergy affects approximately 6.1 million children and adults in the United States.4 The prevalence of peanut allergy has increased from 0.4% (1997) to 2.9% (2021) of the US population and continues to increase.5 Peanut allergy is a substantial health concern in industrialized countries due to an increase in the consumption of diets with immunomodulatory properties. Reasons for the increase include factors other than food allergens per se that increase/decrease reaction to an allergen sometimes by impacting the composition of the gut microbial community, such as reliance on ultra-processed foods, the globalization of dietary habits, and underdeveloped immune systems due to lack of exposure to microorganisms.6 Peanut allergy affects not only the lives of children with sensitivity but also their families by reducing the quality of their lives by impacting them socially, financially, emotionally, and physically.7 The recent landmark LEAP (Learning Early About Peanut) study published in the New England Journal of Medicine showed that the development of peanut allergies can be reduced by more than 80% following exposure to peanut protein early in childhood.8 This resulted in the National Institute of Allergy and Infectious Disease (NIAID) revising its guidelines to recommend infant introduction to peanut protein as early as four to six months of age.9
Standard peanut allergy management involves the complete exclusion of peanuts from the diet, patient education, and the provision of emergency rescue medication.10 Despite vigilance, accidental exposures are common due to cross-contamination and unregulated precautionary labeling.11 Approximately 7% to 14% of peanut-sensitive individuals experience accidental peanut exposure annually.12 Seventeen peanut proteins, Ara h 1 to Ara h 18 (Ara h 4 is renamed Ara h 3.02), are recognized as peanut allergens.13 Among the seventeen peanut allergens, four proteins known as Ara h 1, Ara h 2, Ara h 3, and Ara h 6 are considered major allergens as more than 50% of peanut-sensitive individuals in the United States have IgE (allergen-specific antibodies, proteins the immune system makes to protect the body from foreign elements) recognizing these allergens.14
Peanut allergy is an immune system overreaction to a particular food component. Proteins in everyday food products are nonhazardous to the general population. However, in genetically predisposed individuals, the likelihood of an IgE-mediated hypersensitive response to common food proteins is higher. This risk is also elevated for those who have been exposed to a viral disease early in life or have undergone significant lifestyle changes, such as changes in eating habits or drug/alcohol use, which is often associated with migration or stress.8,15 The immune cells that carry out these responses occur in the gut tissue and bone marrow 16,17 (figure 2). Once the system is exposed to the immunogen (a molecule that triggers an immune response) in sensitive individuals, subsequent exposures, even to trace quantities of the allergen, can lead to a massive allergic reaction, potentially leading to the death of the affected individual.18-21
The allergy is a lifelong burden and is rarely outgrown6. The estimated US caregiver’s cost for peanut allergy is $4.3 billion annually ($724 per child).22 Therefore, there is an unmet need for alternative disease management strategies. Oral *immunotherapy, PalforziaTM, has been recently approved, but it is costly and needs to be regularly administered.23
Allergic Reaction
When an individual ingests food they are allergic to, the incompletely digested protein fragments from the food reach the bloodstream from the gut, where they are recognized by antibodies (proteins that bind to and recognize specific undigested protein fragments or other *biomolecules) bound to white blood cells (immune cells). Antibodies (globular immune protein of type E, Immunoglobulin E or IgE types) induce the white blood cells (such as mast cells) to release histamine (an organic compound involved in immune response communication) and other signaling molecules24 (figure 2).
After receiving the signal, the B-type (bone marrow-derived) white blood cells produce more antibodies. Hence, when a pre-sensitized individual is reexposed to peanut allergens, it crosslinks with specific IgE antibodies on the mast cells and leads to the secretion of different inflammation mediators, such as cytokines (small immune signaling proteins) and histamine, that result in symptom development. Each antibody consists of four protein chains, including two identical heavy chains and two identical light chains. Each chain has a constant and variable region. The variable region (that adopts itself to bind the antigen) forms the surface that physically binds to antigens (e.g., peanut proteins). The constant regions of IgE direct the antibody functions mainly by initiating processes that lead to inflammation.17 Some common manifestations of peanut allergy include skin rash, vomiting, abdominal pain, diarrhea, swelling in the throat, repetitive coughing, wheezing, low blood pressure, and *dysrhythmia.17 (figure 2).
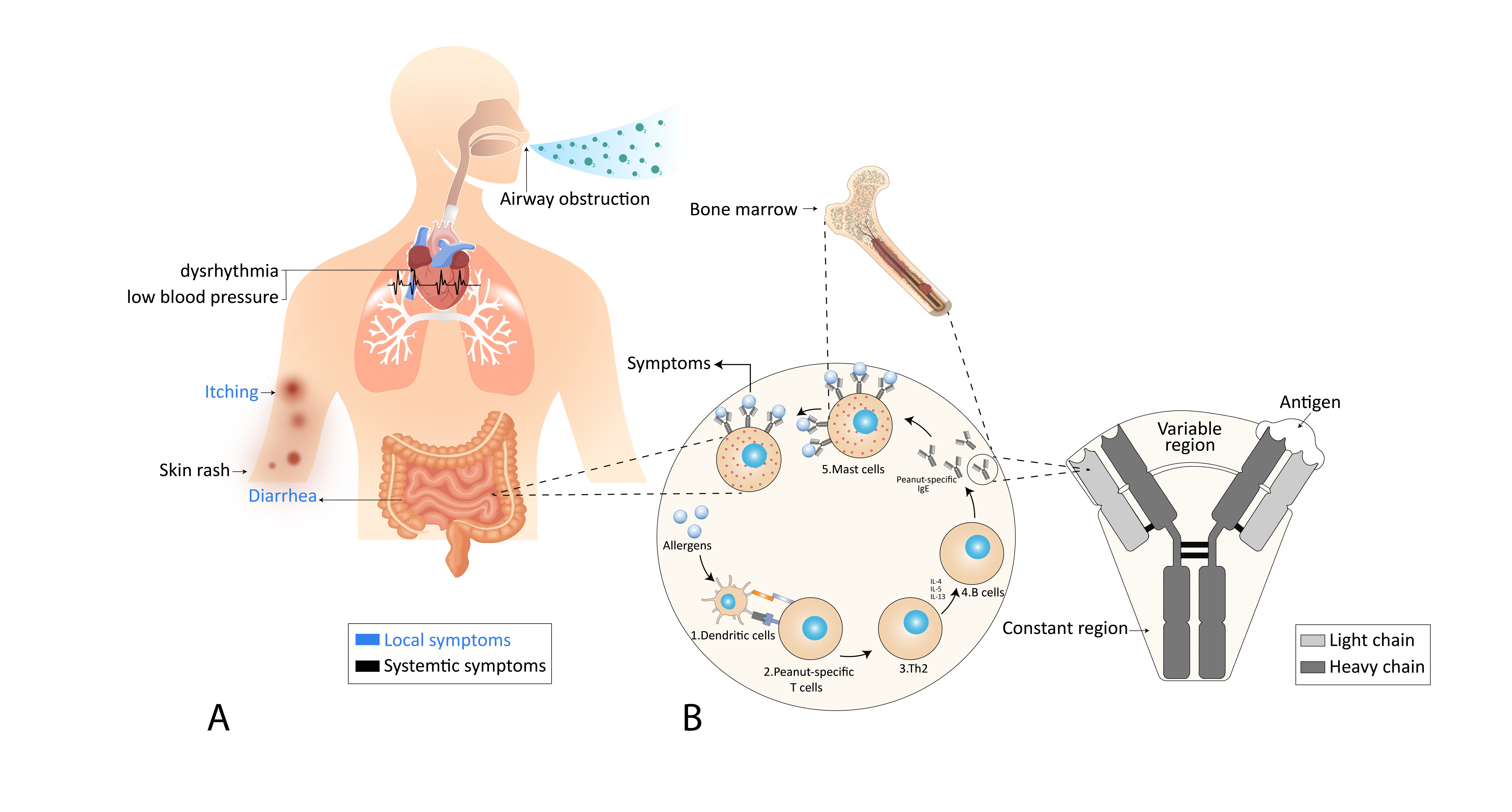
Figure 2. A. Diagrammatic representation of the immune reactions that lead to common peanut allergy symptoms. B. Immunoglobulin E (IgE) antibodies are responsible for allergic reactions to peanut proteins. Bone marrow produces IgE antibodies, and gut tissue locally distributes them. Note: Dendritic cells are immune cells that display the antigen on their surface. T-cells (thymus gland-derived immune cells) are another kind of immune cells that recognize the antigen displayed on the surface of dendritic cells and induce the production of T helper cells (Th2) that secret small cell signaling proteins known as interleukins (ILs), which induce B cells (another kind of immune cells of bone marrow origin) to produce antigen-specific antibodies for antigen recognition in the future exposures. Mast cells are another kind of immune cells that decorate themselves with antibodies to defend against antigens, which, in peanut allergy, are not pathogens but benign food proteins. Image credit: Tariq Alam, Clemson University.
Breeding Reduced-Allergenicity Peanuts
Various efforts to develop peanut allergy treatments are underway. A recently approved oral *immunotherapy is PalforziaTM, which is a precisely measured dose of peanut proteins.23 Except for PalforziaTM, no other immunotherapies have passed the evaluation process. PalforziaTM works in most cases; however, therapy is expensive and cannot be discontinued.
To complement these efforts, hypoallergenic peanut *genotypes are being developed using a *molecular breeding approach. The hypoallergenic genotypes would serve the following purposes:
- Minimize the risk of an intense immune reaction in allergic individuals upon accidental peanut exposure.
- Act as an affordable alternative for PalforziaTM if administered under observation.
- Help build resistance against peanut allergens at an early age if consumed under observation.
Releasing hypoallergenic peanut genotypes will improve the socioeconomics of allergic individuals and their families by reducing the peanut allergy management cost. It would also allow insensitive individuals to enjoy peanuts in public places and settings (e.g., peanut butter served in schools and roasted peanuts offered on airplanes).
At Clemson University, we adopted two strategies to develop reduced allergenicity peanut genotypes.
- Peanut *germplasm screening for reduced content of *immunogenic proteins.
- Site-directed *mutagenesis means at a desired site in the peanut genome (i.e., complete genetic material for reduced content of *immunogenic proteins).
Peanut Germplasm Screening for Reduced Content of *Immunogenic Proteins
We screened the global peanut germplasm collection, including the United States and ICRISAT (International Crops Research Institute for the Semi-Arid Tropics) mini-core collections, and selected South Asian lines for reduced content of allergenic proteins (allergen). We identified several Arachis hypogea genotypes with reduced allergen content.25 Many of the identified genotypes either lacked a specific protein (e.g., genotype ‘P-84A’ lacked a highly *immunogenic peanut protein called Ara h 1) or accumulated a reduced amount of one or more of the four major allergenic proteins, Ara h 1, Ara h 2, Ara h 3, and Ara h 6 (figure 3). We are genetically crossing the selected genotype to produce a line with a stacked protein *phenotype of parental genotypes, either lacking a few major allergenic proteins or accumulating reduced amounts of all four major allergenic proteins (figure 3).
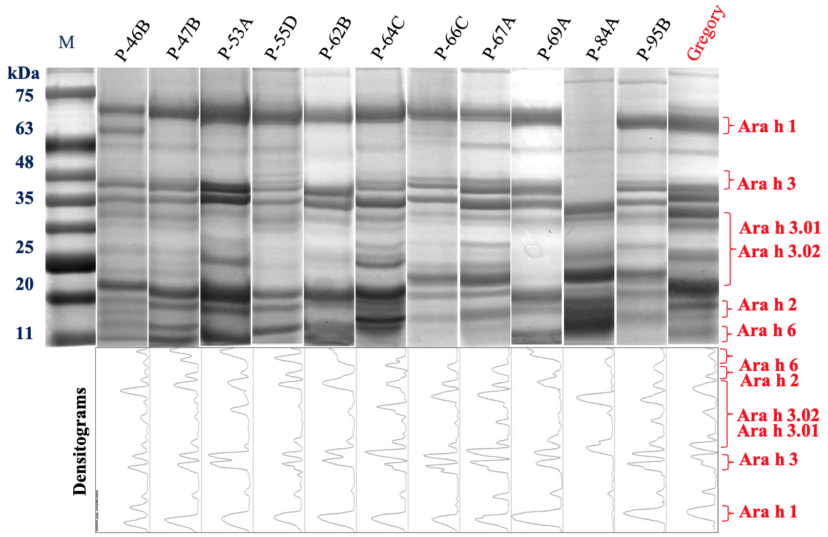
Figure 3. Denaturing-polyacrylamide gel (used to resolve proteins) showing the protein profiles of selected peanut *genotypes with reduced content of major allergenic proteins (Ara h 1, Ara h 2, Ara h 3, and Ara h 6). Notice the missing *immunogenic protein Ara h 1 and Ara h 3.1 bands in the genotype P-84A. Other genotypes have reduced content of different allergens as compared to the reference peanut genotype ‘Gregory.’ The densitograms (optical density of each protein band presented in the form of a peak in the graph) reflect the quantity of different peanut proteins accumulating in each genotype. Notice the missing Ara h 1 and Ara h 3.1 peaks in the P-84A genotype. Also, the peak areas of other genotypes are lower than the reference genotype ‘Gregory.’ M=pre-stained protein marker used to determine the protein sizes on the gel.
Site-Directed Mutagenesis in Peanut for Reduced Content of Immunogenic Proteins
In parallel to the abovementioned strategy, we adopted a *non-transgenic site-directed *mutagenesis approach in peanuts to induce mutations in the genes encoding four major allergenic proteins. Removing all allergens from the peanut seeds is challenging due to their sheer number (seventeen allergenic proteins identified so far) and the polyploid (more than one set of chromosomes) nature of the plant. As mentioned earlier, peanut contains seventeen allergenic proteins, four of which are major allergens. We developed two multigene editing constructs (gene expression constructs that can produce mutations at many desired sites in the genome, which refers to the complete set of genes an organism has) targeting genes for four major allergenic proteins (Ara h 1, Ara h 2, Ara h 3, and Ara h 6) for delivery to plants via particle bombardment (shooting DNA coated gold particles into plant cells) and viral construct (viral DNA modified to carry the gene of interest). We conducted a laboratory-based test to evaluate the performance of genome editing *reagents for their ability to mutate DNA at the target site in the genome before plant delivery. We optimized both *biomolecule delivery methods for developing peanut lines with reduced allergen content (figure 4). Using both delivery approaches, we have developed several *putative mutant lines (figure 4). The developed lines are being tested for mutations at the target site, and the accumulation of allergenic proteins and proteins derived from the selected lines will be evaluated for reduced allergenicity using sera (the clear liquid part of the blood) from peanut-sensitive individuals. Since the genome editing construct will be transiently present in the explants (plant tissue used for tissue culture) or plants infiltrated with disarmed (non-disease causing) viral vectors, the resulting plants will only inherit the mutations induced by these reagents and will be transgene-free.
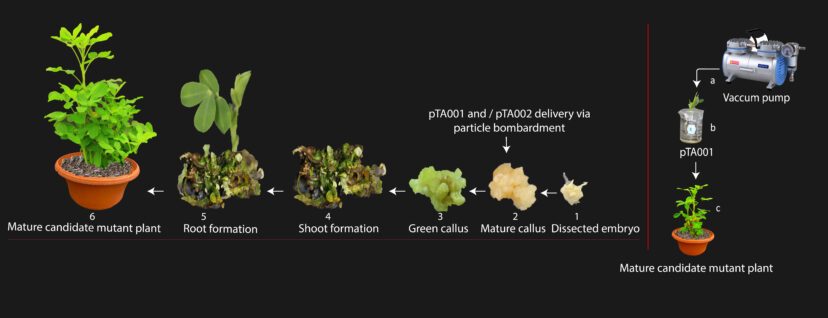
Figure 4. The process of development of hypoallergenic peanut genotypes. Right: a-c, vacuum infiltration of the viral construct and a gene-editing construct pTA001 into the one-week-old plantlets. The vacuum was created via a vacuum pump. After infiltration, the plants were transferred to the soil. Left: 1-6, gold particle-mediated delivery. For this purpose, the embryo (germ) was dissected from mature seeds, the callus (undifferentiated mass of cells) was induced, and subsequently, the gene editing construct, pTA001 or pTA002, was delivered into the callus via particle bombardment; the callus (unorganized mass of plant cells) was transferred to the shoot induction medium and then to root induction media. Later, the mature *putative mutant plant was transferred to the soil. The entire process takes approximately fourteen to sixteen months to produce a mature *putative mutant peanut plant using tissue culture. Image credit: Tariq Alam, Clemson University.
Summary
In summary, this publication informs readers about (1) peanut allergy and reactions susceptible individuals experience upon exposure to abysmal amounts of peanut proteins—eliciting dose, an allergen dose that elicits an immune reaction in 5% of allergic individuals, that equals 1.5 mg peanut proteins or 6 mg whole peanut—through ingestion or other exposure routes, (2) current therapies, and (3) an affordable solution (i.e., hypoallergenic peanut lines) under development at Clemson University.
Glossary of Terms
Biomolecule: Substances produced by cells and living organisms.
Dysrhythmia: Abnormal or irregular heartbeat rhythm.
Genotypes: Genetic makeup of an organism.
Germplasm: Genetic material used to produce new plants.
Hectacre: Equal to 100 acres.
Immunogenic: Relating to or producing an immune response.
Immunotherapy: A treatment that activates or suppresses a person’s immune system.
Molecular Breeding: The use of genetic manipulation (DNA markers) to alter or improve traits.
Mutagenesis: The process of genetic mutations.
Non-Transgenic: Not having been altered with foreign DNA.
Phenotypes: Observable characteristics or traits.
Putative: Commonly accepted or assumed.
Reagents: An added substance that causes a chemical reaction.
Acknowledgments
This work was supported by the SC Peanut Board, the National Peanut Board, and the Foundation for Food and Agriculture Research (FFAR). Further, the authors would like to thank Johanna Staudacher, Amanpreet Brar, Zachary Jones, Rachael Kerr, Brianna Ludlum, and James Kerr for their contributions to the research summarized in the present manuscript.
References Cited
- Mintz S, McNeil S. Centers of Origins Selected Crops. Digital History; 2018 [accessed 2022 Nov 22]. https://www.digitalhistory.uh.edu/active_learning/explorations/columbus/columbian_answers_vegetables.cfm.
- Chenault KD, Ozias‐Akins P, Gallo M, Srivastava P. Peanut. Compendium of transgenic crop plants. 2009;169–198. doi:10.1002/9781405181099.k0204.
- Country Summary. Washington (DC): United States Department of Agriculture (USDA), Foreign Agriculture Service; 2023 [accessed 2022 Nov 22].
https://ipad.fas.usda.gov/countrysummary/Default.aspx?id=US&crop=Peanut. - Facts and Statistics. McLean (VA): Food Allergy Research and Education (FARE); 2023 [accessed 2022 Nov 22,]. https://www.foodallergy.org/resources/facts-and-statistics.
- Warren C, Lei D, Sicherer S, Schleimer R, Gupta R. Prevalence and characteristics of peanut allergy in US adults. Journal of Allergy and Clinical Immunology. 2021;147:2263–2270. doi:10.1016/j.jaci.2020.11.046.
- Potential genetic and environmental determinants of food allergy risk and possible prevention strategies. In: Oria MP, Stallings VA, editors. Finding a path to safety in food allergy: assessment of the global burden, causes, prevention, management, and public policy. Washington (DC): National Academies Press (US), National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee on Food Allergies: Global Burden, Causes, Treatment, Prevention, and Public Policy; 2016 Nov 30. PMID: 28609025.
- Anagnostou K, Clark A. Oral immunotherapy for peanut allergy. Annual Review of Medicine. 2016;67:375–385. doi:10.1146/annurev-med-061014-094943.
- Du Toit G, Roberts G, Sayre PH, Bahnson HT Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. The New England Journal of Medicine. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850.
- Guidelines for clinicians and patients for diagnosis and management of food allergy in the United States. Bethesda (MD): National Institute of Allergy and Infectious Diseases (NIAID); 2018 [accessed 2022 Nov 22]. https://www.niaid.nih.gov/diseases-conditions/guidelines-clinicians-and-patients-food-allergy.
- Sampson HA. Food allergy. Part 2: diagnosis and management. Journal of Allergy and Clinical Immunology. 1999;103(6):981–989. doi:10.1016/S0091-6749(99)70167-3.
- Furlong TJ, DeSimonea J, Sicherer SH. Peanut and tree nut allergic reactions in restaurants and other food establishments. Journal of Allergy and Clinical Immunology. 2001;108(5):867–870. doi:10.1067/mai.2001.119157.
- Lieberman JA, Gupta RS, Knibb RC, Haselkorn T, Tilles S, Mack DP, Pouessel G. The global burden of illness of peanut allergy: A comprehensive literature review. Allergy. 2021;76(5):1367–1384. doi:10.1111/all.14666.
- Palladino C, Breiteneder H. Peanut allergens. Molecular immunology. 2018;100:58–70. doi:10.1016/j.molimm.2018.04.005.
- Shah F, Shi A, Ashley J, Kronfel C, Wang Q, Maleki SJ, Adhikari B, Zhang J. Peanut allergy: characteristics and approaches for mitigation. Comprehensive Reviews in Food Science and Food Safety. 2019;18:1361–1387. doi:10.1111/1541-4337.12472.
- Cheung, DS, Grayson MH. Role of viruses in the development of atopic disease in pediatric patients. Current allergy and asthma reports. 2012;12:613–620. doi:10.1007/s11882-012-0295-y.
- Taylor S. Chemistry and detection of food allergens. Food Technology. 1992;46(146):152.
- Wesemann DR, Nagler CR. Origins of peanut allergy-causing antibodies. Science. 2020;367(6482):1072–1073.
- Wensing M, Penninks AH, Hefle SL et al. The distribution of individual threshold doses eliciting allergic reactions in a population with peanut allergy. Journal of Allergy and Clinical Immunology. 2002;110(6):915–20. doi:10.1067/mai.2002.129235.
- Taylor SL, Moneret-Vautrin DA, Renge WR et al. Threshold dose for peanut: risk characterization based upon diagnostic oral challenge of a series of 286 peanut-allergic individuals. Food and chemical toxicology. 2009;48(3), 814–819. https://doi.org/10.1016/j.fct.2009.12.013.
- Hourihane JO, Kilburn SA, Nordlee JA et al. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebo-controlled food challenge study. Journal of Allergy and Clinical Immunology. 1997;100(5):596–600. doi:10.1016/S0091-6749(97)70161-1.
- Blom WM, Vlieg-Boerstra BJ, Kruizinga AG, et al. Threshold dose distributions for 5 major allergenic foods in children. Journal of Allergy and Clinical Immunology. 2013;131(1):172–79. doi:10.1016/j.jaci.2012.10.034.
- Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatric. 2013;167(11):1026–1031. doi:10.1001/jamapediatrics.2013.2376.
- Abramowicz M, Zuccotti G, Pflomm JM. Peanut allergen powder (Palforzia)(Reprinted from Medical Letter on Drugs and Therapeutics, vol 62, pg 33–34, 2020). JAMA-Journal of the American Medical Association. 2020 Jul 14;324(2):192–193. doi:10.1001/jama.2020.3599.
- Couzin-Frankel J. Toxin or treatment? Science. 2018;278–282. doi:10.1126/science.362.6412.278.
- Rustgi S, Alam T, Jones ZT, Brar AK, Kashyap S. Reduced-immunogenicity wheat and peanut lines for people with foodborne disorders. Chemistry Proceedings. 2022;10(1):67. doi:10.3390/IOCAG2022-12221.

