Sugarberry (Celtis laevigata), commonly called southern hackberry in the southern US, is a medium- to large-sized native tree found along floodplains and rivers in the Southeast. In some areas, sugarberry can be found in parks, yards, and along fences and streets. Hackberry (Celtis occidentalis) grows more commonly in the northern part of sugarberry’s range and extends through the Midwest and northeastern areas of the United States. Both species grow throughout the eastern United States and are occasionally used as ornamentals.1 Though often overlooked, the Society of Municipal Arborists has named hackberry the 2020 Urban Tree of the Year. The small fruit of Celtis trees provides food to many wildlife species like birds and small game. The foliage is the only food source for caterpillars of several butterfly species, such as the tawny emperor butterfly (Asterocampa clyton), American snout butterfly (Libytheana carinenta), and the hackberry emperor butterfly (Asterocampa celtis).2 Sugarberry and hackberry wood is desired for its light color and is also used for furniture, flooring, pallets, and pulp.1

Figure 1. Declining sugarberry tree (Celtis laevigata) near North Augusta, SC, typical of many seen experiencing sugarberry decline in this region. Note the sparse canopy and branch dieback compared to healthy, green trees nearby. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station.
Sugarberry has been declining and dying at elevated rates in South Carolina and Georgia over the past decade (figure 1). In recent years, cities experiencing dying sugarberry have removed many valuable shade and street trees, due to associated risks with dead limbs and trees. While the causal issue has not yet been identified, a woodboring beetle, the flatheaded hackberry borer (Agrilus macer), is commonly associated with declining and dying sugarberry trees in this region. This beetle is native to the US and develops in stressed sugarberry and hackberry trees in urban, suburban, and forested settings. While the presence of flatheaded hackberry borers in any life stage can be an indicator of tree stress and may hasten the death of weakened sugarberry trees, this beetle does not appear to be a primary cause of tree decline or mortality. Under favorable conditions, trees can overcome moderate attacks from this conspicuous secondary pest.3
Species related to the flatheaded hackberry borer, such as the two-lined chestnut borer (A. bilineatus) and the bronze birch borer (A. anxius), can also cause tree damage and losses within their native range. Other related species are emerging pests and highlight the damaging capabilities of Agrilus species. The emerald ash borer (A. planipennis) is one of the most impactful forest pests in North America, and the two-spotted oak borer (A. biguttatus) is commonly associated with oak decline in Europe.
Description and Life History
The flatheaded hackberry borer belongs to the family of flatheaded borers (Buprestidae), which are commonly characterized by elongated, “bullet-shaped” bodies (figure 2a). Adult flatheaded hackberry borers are metallic black to dark green in color and 1/3-2/3 inches (8-16 mm) in length (figure 2b). Adults can be easily distinguished from other native Agrilus species by the raised ridge on each outer wing cover.4

Figure 2a. The flatheaded hackberry borer is a small, brownish beetle with short antennae and a light tan colored underside. Here it is shown laying a round, beige egg mass on a tree. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station and E.M. Poole, University of Georgia, Department of Entomology.

Figure 2c. Flatheaded hackberry borer egg masses are round, beige to tan in color, and raised off the bark surface. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station and E.M. Poole, University of Georgia, Department of Entomology.
Adults are most active in the summer months and can be found on sugarberry and hackberry foliage or on the trunks of weakened trees. After mating, females will lay clusters of eggs (figure 2a), each with an average of 16 eggs, on the smooth areas of sugarberry and hackberry trunks and branches. The female then covers the egg clusters with a green substance and smooths the surface with a final tan substance that hardens into a distinctive cap, presumably to protect the egg mass from moisture loss and predators such as ants (figure 2c). Older egg masses turn from a light beige or tan to a brown color. The egg masses appear as bumps on the bark of the tree and can be present at incredible densities from the tree base to the ends of branches (figure 3a).

Figure 3a. A felled sugarberry covered with light-colored flatheaded hackberry borer egg masses. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station.
The large egg masses produced by the flatheaded hackberry borer are unusual, but not unheard of, among flatheaded beetles and may be a tactic for overcoming local host-plant defenses. Upon hatching, larvae bore through the bark and begin excavating galleries in the phloem, cambium, and outer sapwood (figure 4a–d). Extensive larval boring can girdle trees and are the primary cause of damage, as the boring limits the movement of sugars and nutrients within the tree. Healthy trees can withstand A. macer larval feeding and boring and will respond by forming callus tissue at the site of the egg mass and hatched larvae (figure 3b, c). After larval development, emerging adults will leave small D-shaped exit holes, approximately 1/8-1/4 inches (3.2-6.4) in width, along the trunk and main branches of the trees (figure 5).3
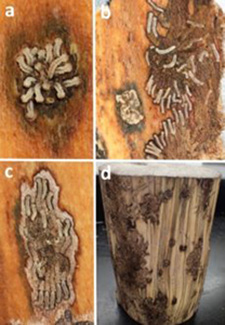
Figure 4. (a) Flatheaded hackberry borer larvae from a single egg mass as seen from the underside of bark. (b, c) Feeding immature flatheaded hackberry borer on the underside of bark. (d) A de-barked log showing multiple colonization points and associated xylem discoloration. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station.

Figure 5. D-shaped exit hole on the trunk of a sugarberry tree, left when an adult flatheaded hackberry borer exits the tree after it completes development. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station.
Distribution and Hosts
Flatheaded hackberry borers (figure 6a) can be found from California in the West to South Carolina in the East. Six native Celtis species occur across the range of the flatheaded hackberry borer, and all are potential hosts, with sugarberry (C. laevigata) and hackberry (C. occidentalis) being the most widespread species in the eastern US.5 These tree species have smooth bark when young (figure 6b) but are known for their warty bark on older trees (figure 6c). Mature trees have round fruit (figure 6d), which ripens to shades of orange or red, and ovate leaves that are variable but often serrated along leaf edges (figure 6e). In addition to these native species, a species of non-native hackberry (e.g., Chinese hackberry, C. sinensis) has become naturalized in North America.6
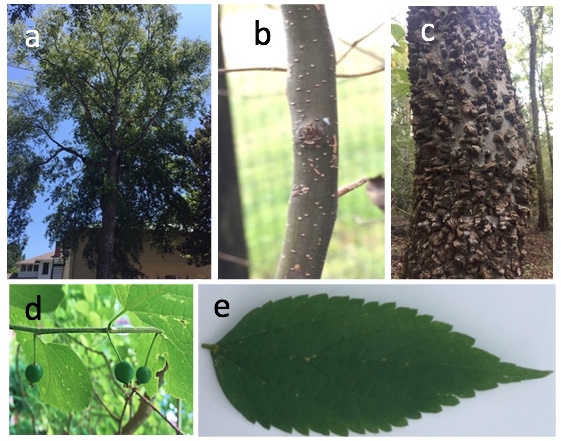
Figure 6. (a) A healthy sugarberry (C. laevigata) with a full green canopy. (b) Young sugarberry trees have smooth greyish bark with little white bumps that will later become knobby, wart-like structures. (c) Older trees have a warty textured bark with many raised bumps. (d) Sugarberry has small round fruits. (e) Healthy sugarberry leaves often have serrated or ridged edges. Image credit: E.M. Poole, University of Georgia, Department of Entomology.
Damage and Impact
Flatheaded hackberry borer galleries can be visible beneath the bark, and attacked trees may weep black liquid around the egg masses (figure 7). Discoloration can be seen around the galleries of the larvae, but this color pattern is most likely due to weakly pathogenic fungi that cause a very limited host response. There is no evidence flatheaded hackberry borers transmit highly pathogenic fungi.3

Figure 7. When flatheaded hackberry borer egg masses hatch, the tree often responds by oozing or weeping a black fluid that can result in the trunk of a heavily infested tree looking like it is covered in black spots. Image credit: M.D. Ulyshen, USDA Forest Service, Southern Research Station.
Flatheaded hackberry borers appear to be relatively rare throughout most of its range, but significant population increases can occur in areas experiencing high rates of Celtis mortality. Under such situations, attacks by this species are likely to further weaken trees and have the potential to hasten their death. In recent years, parts of South Carolina and Georgia have experienced severe sugarberry mortality with associated symptoms of small yellowing leaves, premature defoliation, and branch dieback, and the flatheaded hackberry borer is active throughout this area.3
Prevention and Management
At this time, no effective management strategies are known, although chemical treatments (e.g., those with the active ingredients emamectin benzoate, dinotefuran, azadirachtin, or imidacloprid) are known to be useful in protecting against other species of Agrilus, such as the emerald ash borer.7 However, because the flatheaded hackberry borer is not a primary cause of Celtis mortality, trees attacked by this species are likely to be experiencing one or more other stressors that predisposed them to attack by the flatheaded hackberry borer.3
Acknowledgments
Thanks to Jennifer Moore-Myers (USDA-Forest Service), Kevin Chase (Bartlett Tree Experts), and our three anonymous reviewers for comments on an earlier version of this document.
References Cited
- Tirmenstein D. Celtis laevigata var. reticulata. Fort Collins (CO): US Department of Agriculture Forest Service, Fire Effects Information System, Rocky Mountain Research Station, Fire Sciences Laboratory. 1990 [accessed 2020 Mar 13]. https://www.fs.fed.us/database/feis/plants/tree/cellaer/all.html.
- Minno M, Butler J, Hall, D. Florida Butterfly caterpillars and their host plants. Gainesville (FL): University of Florida; 2005.
- Poole EM, Ulyshen MD, Horn S, Cram M, Olatinwo R, Fraedrich S. Biology and distribution of Agrilus macer LeConte (Coleoptera: Buprestidae), a species associated with sugarberry (Celtis laevigata Willd.) mortality in the southeastern USA. Ann For Sci. 2019;76(1):7. doi:10.1007/s13595-018-0794-7.
- Harpootlian PJ, Bellamy CL. Jewel beetles (Coleoptera: Buprestidae) of South Carolina. Clemson (SC): Clemson University; 2014.
- Ford AL, Van Auken OW. The distribution of woody species in the Guadalupe River floodplain forest in the Edwards Plateau of Texas. Southwest Nat. 1982;27:383–392. doi:10.2307/3670713.
- Whittemore AT. Celtis, in Jepson Flora Project. Berkeley (CA): The Jepson Herbarium, University of Berkeley, Jepson eFlora; 2012. http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=10750.
- Herms D, McCullough D, Smitley D, Sadof C, Miller F, Cranshaw W. Insecticide options for protecting ash trees from emerald ash borer. 3rd ed. East Lansing (MI): North Central IPM Center Bulletin; 2019. 16 p.




